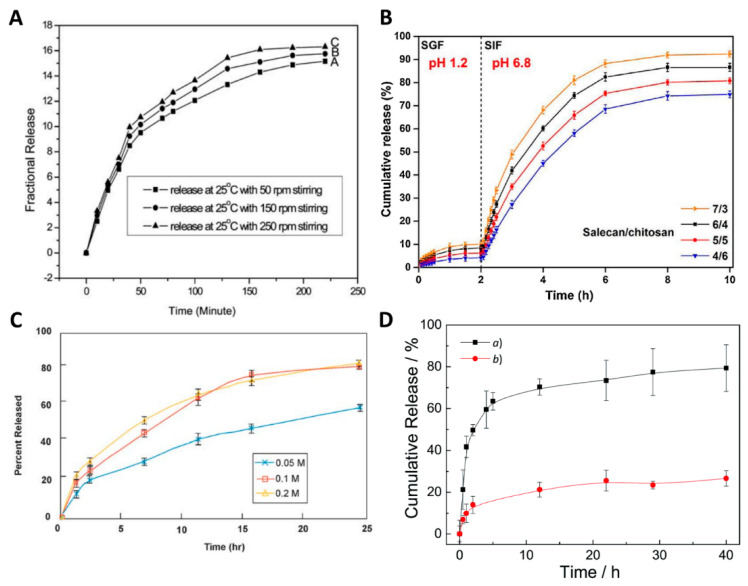
Figure 2.
Parameters influencing in vitro drug release. (A) Release of indomethacin from a hydrogel system at 25 °C with different stirring rates (50 rpm, 150 rpm, 250 rpm). Reproduced with permission from [35]. Copyright the Royal Society of Chemistry, 2010. (B) Cumulative release (%) of Vitamin C from Salecan/chitosan polyelectrolyte complex hydrogels at different hydrogel ratio in simulated gastric fluid (SGF) for the first two hours and simulated intestinal fluid (SIF) from the second hour until the end of the release assay. Reproduced with permission from [12]. Copyright Elsevier, 2020. (C) Release of Verapamil in phosphate buffers at different molarities (0.05 M, 0.1 M, and 0.2 M). Data are shown in means ± standard deviation, n = 6. Reproduced with permission from [113]. Copyright Dissolution Technologies, 2007. (D) In vitro release of Bufalin at 10 mM PBS, pH 7.4, 37 °C in the (a) presence, and (b) absence of esterase (10 units). Reproduced with permission from [37]. Copyright the Royal Society of Chemistry, 2016.