Abstract
Background
The demand for traditional herbal medicine is increasing and about 85% of the world population use herbal medicines for the prevention and treatment of diseases. More than 62.5% of the forest areas in Ethiopia are found in the southwest region, which have been used as a source of traditional medicine to treat different human and livestock ailments. The aim of this study was the investigation of the ethnobotanical and physicochemical properties of commonly used medicinal plants in Jimma zone, southwest Ethiopia.
Materials and Methods
A cross-sectional study was conducted in the district of Jimma zone from June 1 to 30, 2017. The ethnobotanical data were collected from traditional healers through semi-structured questionnaires. Specimens collected from various habitats were taken into Jimma University, Herbarium laboratory, dried, and prepared using standard herbarium specimen techniques for identification. Physicochemical analysis was done for selected medicinal plants.
Results
A total of 72 medicinal plants categorized under 61 genera and 39 families were stated by the respondents for the treatment of different human and livestock ailments. Herbs constitute the largest category (28 species, 38.89%) followed by shrubs (21 species, 29.17%), trees (20 species, 27.78%) and climbers (3 species, 4.17%). Leaves (39.19%) were the most commonly used plant parts followed by roots (27%) and seeds (10.81%). Traditional healers reported processing remedies mainly through crushing (46.91%), powdering (18.52%), pounding (11.11%), and pressing (9.88%). The water-soluble extractive value of the selected medicinal plants were between 1.825 to 18.507%w/w and the alcohol-soluble extractive value were between 0.143 to 1.107%w/w. The moisture content (%LOD) of Barleria argentea Balf. f. was higher than the recommended standard which consisted of 21.063%w/w and followed by high %LOD of Crateva adansonii D.C. Prodr (8.143%w/w) and Euphorbia abbyssinica J.F. Gmel. (16.347%w/w). The highest total ash value was registered in Dioscorea quartiniana A.Rich. species which consisted of 18.563%w/w and followed by Crateva adansonii D.C. Prodr (16.033%w/w) and Barleria argentea Balf. f. (15.648%w/w). High acid-insoluble ash value (7.227%w/w) and water-soluble ash (6.731%w/w) was recorded in Euphorbia abbyssinica J.F. Gmel.
Conclusion
The study revealed that the water-soluble extractive value of the selected medicinal plants indicates the presence of water-soluble components such as sugar, acids, and inorganic compounds. In the future, these characters can be used to check the genuine nature of the crude drug; thus, it plays an important role in preventing the possible steps of adulteration.
Keywords: botanical diversity, extractable matter, ash values, moisture content, medicinal plants
Introduction
Medicinal plants have become an alternative source of remedy for many people all over the world and serve as one of the potential sources for discovery of various active compounds. Pharmaceutical importance of plants has led to exploration of plant extracts which are commonly used in traditional medicine.1 Approximately 85%2 of the population use medicinal plants for avoidance and management of diseases, and the demand is increasing in developed and developing countries. Drugs containing compounds obtained from higher plants account for 25%. Only 10% of medicinal plant species are cultivated today while the majority are at risk in the wild.3
In many parts of the world where herbal medicines are widely used, certain factors have militated against their inclusion in the mainstream health-care system include poor dosage form design and standardization of products.4 In recent times there has been an upsurge in the use of herbal medicines, which has become more conventional due to improvement in regulation, analytical and quality control tools as well as advances in clinical research showing great value in general health-care and management of certain diseases in which conventional medicines have not done too well.5
Herbal medicines are frequently considered to be safer than conventional medicines because of their better tolerance. But there are reports of side effects and adverse reactions that have been related to herbal medicines.6 Some of these side effects and adverse reactions are due to the intrinsic bioactive secondary metabolites present in the herbal materials. In addition, poor quality of the products is attributed to contamination (with chemicals, pesticides, microorganisms, and heavy metals), adulteration with pure drug compounds and poor quality control measures. Therefore an acceptable herbal product must be safe, stable and presented in a suitable dosage form and package.7
Ethiopia is endowed with diverse biological resources including about 6,500 species of higher plants, with approximately 12% endemic, hence making it one of the six highest plant biodiversity rich regions. More than 62.5% of its forest areas are found in the southwest region of Ethiopia where most of the medicinal plants are confined and have been used as a source of traditional medicine to treat different human and livestock ailments.3
Strict quality control of traditional medicine is based largely on the empirical measurement of a few nonspecific physical properties such as pH, viscosity, density etc. But quality control by specific methods still requires authenticity. This problem lies in the process of manufacture of the traditional drugs. According to the WHO cited by Annan et al. 2013, to ensure reproducible quality of herbal plants (or preparations), physicochemical and phytochemical characterizations are required to be carried out for establishing their identity, purity, and quality standards.8 Physicochemical standards were generally used for deciding the identity, purity and strength of the drug source. These parameters were also used to detect the adulterants if present in the plant material. Physicochemical information could form the basis for further research towards drug discovery and development from medicinal plants.
Therefore, there is a need for documentation of physicochemical investigations for profiling the quality control parameters of medicinal plants/plant-derived crude/herbal drugs. It was revealed that no reports were available on the physicochemical quality of medicinal plants in Ethiopia. Thus, the aim of the current study was investigation of ethnobotanical and physicochemical properties of commonly used medicinal plants in Jimma zone, southwest Ethiopia.
Materials and Methods
Study Area and Study Design
The plant materials were obtained from the Jimma zone, southwest Ethiopia. A map of Jimma zone, Oromia Regional State is presented in Figure 1. Traditional healers found in these zones were identified and selected through contacting community leaders and the administrative staff of the zone. The traditional healers were interviewed to identify the most commonly used medicinal plants/parts/preparation. The plant samples were collected and transported to Jimma University Herbarium laboratory for species identification. Physicochemical analyses of samples were conducted at Jimma University Laboratory of Drug Quality (JULaDQ) and Jimma University, School Pharmacy, Pharmacognosy Laboratory.
Figure 1.
The Map of Jimma zone, Oromia Regional State.
Note: Adapted from Copyright ©2019 Belew S, Suleman S, Mohammed T, et al. Quality of fixed dose artemether/lumefantrine products in Jimma Zone, Ethiopia. Malar J.2019;18:236. doi:10.1186/s12936-019-2872-1. 9
A cross-sectional study was conducted in selected district of Jimma Zone from June 1 to 30, 2017. The study sites and kebeles (neighborhoods) were selected based on the prior information gathered from community leaders, knowledgeable elders, especially indigenous resourceful elder people greater than 40 years, health workers, and a number of traditional healers in the area.
Data Collection and Analysis
Purposeful sampling technique was used for sample survey selection. A total of 82 informants (traditional healers), individuals in the age range of 40 and above (52 male and 30 female), were randomly selected from 12 districts and out of these, 10 key informants (6 male and 4 female medicinal plant practitioners) were selected purposefully with the help of local administrators and community leader from each district. The ethnobotanical data were collected from traditional healers through a semi-structure questionnaire. Group discussion and field observation were also employed to collect ethnobotanical information. Group discussions were conducted to obtain details of the popular medicinal plants, preservation methods, and method of preparation of medicinal plants in the study area. Every traditional healer was asked to list the main diseases he/she treated and the plants used in the treatment, preparation methods, parts used and route of applications. Field walks with traditional healers were employed to collect specimens of each medicinal plant species. Medicinal plant samples were collected from traditional healers’ own gardens as well as from field (wild). Based on ethnobotanical information provided by informants, specimens were collected, numbered, pressed, and dried for identification. The completeness of the data was checked. Errors related to inconsistency were verified using cross tabulation and other data exploration methods. The data were exported to Microsoft Excel, then recoded, categorized and sorted to facilitate analysis. The data were analyzed using Microsoft Excel.
Plant Collection
The reported medicinal plants were collected from natural vegetation (forest) or home gardens with the help of the traditional healers. The collected plant specimens were dried, identified, and deposited at Jimma University Herbarium. Identification of plants were performed by taxonomists using standard procedures. The specimens were identified by comparison with already identified specimens and using taxonomic literatures. All plant names were authenticated using www.worldfloraonline.org or theplantlist.org.
Preference Ranking
Preference ranking was used to rank the popular medicinal plants and medicinal plants used in the treatment of particular ailments in the study area. Each variable was ranked by 10 selected informants. All informants were oriented on each variable and asked to mark the highest value (10) for most preferred and the lowest value (1) for the least preferred on preparation methods, degree of popular medicinal plants and the healing potential of individual medicinal plants. Finally, the values was summed up, ranked and illustrated using tables.
Fidelity Level Index
Fidelity level index (FL) is used to quantify the importance of a given species for a particular purpose in a given cultural group. In this study, FL was used to determine the relative healing potential of 22 medicinal plants against 4 human ailments based on the proportion of informants’ agreement on the use of a given medicinal plant against a given ailment category. The formula used was as follows:
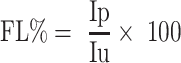 |
FL% is Percentage of Fidelity Level; Ipis the number of informants that indicated the use of a specific plant to treat a disease category; Iu represents the total number of informants that have confirmed the use of the plant for any category of diseases. High fidelity value is indicative of the frequency of use of a particular plant to treat a specific disease.10
Informant Consensus Factor (ICF)
ICF was calculated according to the previously described method11 and used to determine the similarity of the types of plants used by herbal practitioners in treating various categories of diseases. An ICF value close to 0 or 1 indicates that the choice of plant for a diseases category is random or that the majority of herbal practitioners agree on the use of plants for a particular disease, respectively.
ICF=Nur-Nt/Nur-1
Where Nur is the number of uses reported for a particular disease category, Nt indicates the number of taxa used for a particular disease.
Physicochemical Investigations
Based on the degree of agreement on ranking of 10 medicinal plants and the principle that the plant species are widely known and frequently used for the treatment of a particular ailment among the local community, six medicinal plants were selected for further processing for physicochemical investigation. Physicochemical investigations of each prepared sample was carried out for the determination of total ash, water-soluble ash, acid-insoluble ash, water- and alcohol-soluble extractive values and moisture content which was determined by loss on drying (LOD) method. Physicochemical investigations were performed using WHO standard procedures.12 Each study was performed in triplicate and mean values with standard deviation (SD) were calculated.
Results
Botanical Diversity
A total of 72 medicinal plants were stated by the respondents for the treatment of different human and livestock ailments and their specimens were collected from the traditional healers’ own gardens and from forests. The collected specimens were identified based on herbal standard preparation procedures and categorized under 61 genera and 39 families (Table 1). Family Fabaceae was the largest medicinal plant family used for the treatment of different diseases in the study area, and represented 11 species (15.28%). Family Euphorbiaceae was the second most used group of medicinal plants by the community next to the Fabaceae and consisted of five species (6.94%), followed by Lamiaceae, and Asteraceae which consisted of four species each (5.56%) (Table 2). The presence of such a large number of medicinal plant species and associated ethnomedicinal knowledge in the zone indicates that the area has a very high diversity of medicinal plant species and is a site for varied indigenous knowledge. Analysis of growth forms of these medicinal plants revealed that herbs constitute the largest category (28 species, 38.89%) followed by shrubs (21 species, 29.17%), trees (20 species, 27.78%), and climbers (3 species, 4.17%).
Table 1.
Botanical Identification of Medicinal Plants Collected from Traditional Healers in Jimma zone, Southwest Ethiopia
| Family | Scientific Name | Local Names | Growth Habit | Voucher No |
|---|---|---|---|---|
| Acanthaceae | Asystasia guttata (Forssk.) Brummitt | Baquli | Herb | JS101 |
| Acanthaceae | Barleria argentea Balf. f. | Hokto | Shrub | JS79 |
| Acanthaceae | Barleria eranthemoides R. | Shishi | Shrub | JS80 |
| Alliaceae | Allium sativum L. | Qullubbii | Herb | JS75 |
| Aloaceae | Aloe monticola Reynolds | Hernias | Herb | JS 3 |
| Aloaceae | Aloe macrocarpa Tod | Dheri | Herb | JS 76 |
| Amaranthaceae | Amaranthus caudatus L. | Iyyaasuu | Herb | JS102 |
| Amaranthaceae | Achyranthes aspera L. | Jibir | Herb | JS70 |
| Apocynaceae | Carissa edulis Vahl | Akama | Shrub | JS 16 |
| Apocynaceae | Carissa spinarum L. | Hagamsa | Shrub | JS 24 |
| Asteraceae | Acmella caulirhiza Del. | Gutichaa | Herb | JS 21 |
| Asteraceae | Artemisia abyssinica Sch.Bip.ex.Rich/ | Ariti | Herb | JS 35 |
| Asteraceae | Echinops kebericho, Mesfin | Qabarichoo | Herb | JS 77 |
| Asteraceae | Guizotia scabra (Vis.) Chiov. | Adaa | Shrub | JS 53 |
| Balanitaceae | Balanites aegyptica (L.) Del. | Badanna | Tree | JS83 |
| Boraginaceae | Cynoglossum amplifolium Hochst. ex DC. | Karchaba | Herb | JS103 |
| Brassicaceae | Coronopus didymus (L.) Sm | Surumaa | Shrub | JS 25 |
| Cactaceae | Opuntia ficus-indica (L.) Mill. | Nimi | Tree | JS 38 |
| Capparidaceae | Crateva adansonii D.C. Prodr | Qolladii | Shrub | JS 04 |
| Capparidaceae | Ritchiea albersii Gilg | Arbuu | Tree | JS 27 |
| Capparidaceae | Cadaba farinose Forssk. | Galee | Shrub | JS05 |
| Celasteraceae | Catha edulis (Vahl) | Chati | Tree | JS112 |
| Chenopodiaceae | Chenopodium ambroisoides L. | Ajo | Herb | JS105 |
| Combretaceae | Combertum paniculatum Schinz | Baggii | Shrub | JS 07 |
| Crassulaceae | Kalanchoe densiflora Rolfe | Endahula | Herb | JS 61 |
| Cucurbitaceae | Coccinia grandis (L.) Voigt | Gale | Herb | JS111 |
| Cucurbitaceae | Cucumis dipsaceus Ehrenb. ex Spach | Kurera | Herb | JS89 |
| Cucurbitaceae | Cucurbita pepo L. | Buqqee | Herb | JS 06 |
| Dioscoreaceae | Dioscorea quartiniana A. Rich. | Gishu | Tree | JS104 |
| Dracenaceae | Sansevieria ehrenbergii Schweinf. ex Bak. | Muka koricha | Herb | JS114 |
| Euphorbiaceae | Croton macrostachyus Del. | Bakkannisa | Tree | JS 38 |
| Euphorbiaceae | Euphorbia abbyssinica J.F. Gmel. | Adaamii | Tree | JS36 |
| Euphorbiaceae | Euphorbia dumalis S. Carter | Dargu adi | Shrub | JS115 |
| Euphorbiaceae | Euphorbia heterophylla L. | Ananole | Herb | JS123 |
| Euphorbiaceae | Justicia schimperiana (Nees) T. Anderson | Loomii | Tree | JS 42 |
| Fabaceae | Acacia abyssinica Hochst ex.Benth. | Laaftoo | Tree | JS 26 |
| Fabaceae | Acacia hockii De Wild | Chaqkent | Shrub | JS 62 |
| Fabaceae | Acacia mellifera (Vahl) Benth. | Dille | Shrub | JS72 |
| Fabaceae | Albizia anthelmintica Brongn. | Dheta | Tree | JS69 |
| Fabaceae | Albizia schimperiana Oliv. | Imalaa | Tree | JS 65 |
| Fabaceae | Caesalpinia volkensii Harms | Sidika | Climber | JS107 |
| Fabaceae | Calpurnia subdecandra (L’Herit.) Schweick. | Ceeqaa | Shrub | JS 28 |
| Fabaceae | Erythrina abyssinica Lam. Ex. DC. | Beroo | Tree | JS 63 |
| Fabaceae | Indigofera dauensis Gillett | Korsa Birti | Herb | JS08 |
| Fabaceae | Pterolobium stellatum (Forssk.) | Kejima | Climber | JS119 |
| Fabaceae | Taverniera abyssinica A. Rich. | Dingatagna | Shrub | JS 18 |
| Lamiaceae | Ajuga integrifolia Buch.-Hamn. | Armaguusa | Herb | JS 29 |
| Lamiaceae | Clerdendrum myricoides Hochst | Maraasisa | Shrub | JS 32 |
| Lamiaceae | Ocimum gratissimum L. | Damakase | Shrub | JS11 |
| Lamiaceae | Ocimium lamifolium Hochst. Ex. Benth. | Hancabbii | Herb | JS 15 |
| Lauraceae | Cassytha filliformis L. | Korsa buda | Climber | JS122 |
| Loganiaceae | Buddleja polystachya Fresen. | Hanfaaree | Tree | JS 31 |
| Melianthaceae | Bersama abyssinica Fresen. | Lolchiisaa | Shrub | JS 12 |
| Moraceae | Ficus sycomorus L. | Odaa | Tree | JS 85 |
| Muluginaceae | Glinus lotoides L. | Mataharree | Herb | JS81 |
| Myrsinaceae | Maisa lanceolata Forssk. | Abbayii | Tree | JS90 |
| Myrtaceae | Eucalyptus globules labing | Bargamoo Adii | Tree | JS 48 |
| Poaceae | Cynodon dactylon L. Pers. | Coqorsa adii | Herb | JS 30 |
| Poaceae | Cynodon nlemfuensis Vanderyst | Coqorsa gurraacha | Herb | JS 46 |
| Phytolaccaceae | Phytolocca dodecandra L ‘Hert. | Andoodee | Herb | JS 34 |
| Plumbaginaceae | Plumbago zeylanica L. | Martus | Herb | JS 41 |
| Polygalaceae | Rumex neppalensis Spreng | Tult | Herb | JS 10 |
| Polygalaceae | Securidica longipedunculata Fresen | Etsamanaay | Tree | JS 13 |
| Ranunculaceae | Nigella sativa L. | Gurra | Shrub | JS 22 |
| Rocaceae | Prunus africana (Hook. f.) Kalkman | Hoomii | Tree | JS 37 |
| Rubiaceae | Coffee arabica L. | Buna | Tree | JS20 |
| Rutaceae | Clausena anisata (Wild.) Benth. | Ulumaa’i | Tree | JS 39 |
| Rutaceae | Ruta chalepensis L. | Cilaattama | Herb | JS 44 |
| Simarobouceae | Brucea antidysentrica J.F.Mill. | Qomonyo | Shrub | JS47 |
| Solanaceae | Datura stramonium L. | Asaangira | Shrub | JS 56 |
| Solanaceae | Withania somnifera L. Dunal | Kumo | Shrub | JS 55 |
| Zingibiraceae | Zingiber officinale Roscoe | Zinjibila | Herb | JS17 |
Table 2.
Medicinal Plant Families in the Study Area with the Corresponding Numbers of Species
| S.No | Families | Numbers of the Species | Percentage of the Species |
|---|---|---|---|
| 1 | Acanthaceae | 3 | 4.17% |
| 2 | Alliaceae | 1 | 1.39% |
| 3 | Aloaceae | 2 | 2.78% |
| 4 | Amaranthaceae | 2 | 2.78% |
| 5 | Apocynaceae | 2 | 2.78% |
| 6 | Asteraceae | 4 | 5.56% |
| 7 | Balanitaceae | 1 | 1.39% |
| 8 | Boraginaceae | 1 | 1.39% |
| 9 | Brassicaceae | 1 | 1.39% |
| 10 | Cactaceae | 1 | 1.39% |
| 11 | Capparidaceae | 3 | 4.17% |
| 12 | Celasteraceae | 1 | 1.39% |
| 13 | Chenopodiaceae | 1 | 1.39% |
| 14 | Combretaceae | 1 | 1.39% |
| 15 | Crassulaceae | 1 | 1.39% |
| 16 | Cucurbitaceae | 3 | 4.17% |
| 17 | Dioscoreaceae | 1 | 1.39% |
| 18 | Dracenaceae | 1 | 1.39% |
| 19 | Euphorbiaceae | 5 | 6.94% |
| 20 | Fabaceae | 11 | 15.28% |
| 21 | Lamiaceae | 4 | 5.56% |
| 22 | Lauraceae | 1 | 1.39% |
| 23 | Loganiaceae | 1 | 1.39% |
| 24 | Melianthaceae | 1 | 1.39% |
| 25 | Moraceae | 1 | 1.39% |
| 26 | Muluginaceae | 1 | 1.39% |
| 27 | Myrsinaceae | 1 | 1.39% |
| 28 | Myrtaceae | 1 | 1.39% |
| 29 | Poaceae | 2 | 2.78% |
| 30 | Phytolaccaceae | 1 | 1.39% |
| 31 | Plumbaginaceae | 1 | 1.39% |
| 32 | Polygalaceae | 2 | 2.78% |
| 33 | Ranunculaceae | 1 | 1.39% |
| 34 | Rocaceae | 1 | 1.39% |
| 35 | Rubiaceae | 1 | 1.39% |
| 36 | Rutaceae | 2 | 2.78% |
| 37 | Simarobouceae | 1 | 1.39% |
| 38 | Solanaceae | 2 | 2.78% |
| 39 | Zingibiraceae | 1 | 1.39% |
| Total | 72 |
Plant Parts Used
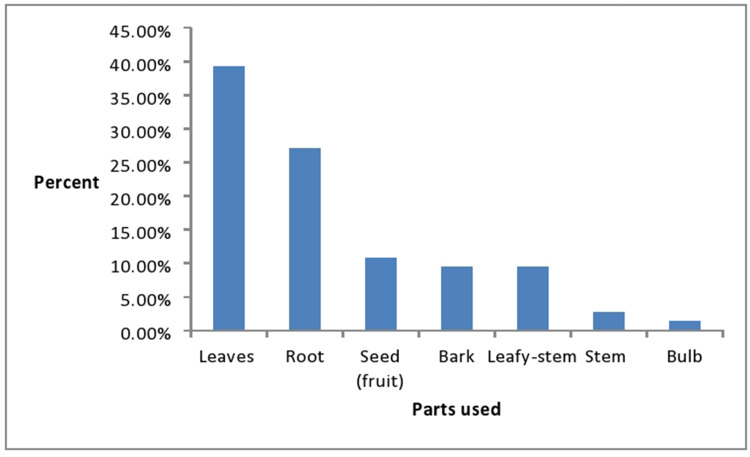
The medicinal plant parts used widely to treat human and livestock ailments include leaves, root, stem, and others. The most commonly used medicinal plant parts for the preparations of the medicines into different dosage forms in the study area were leaves which consisted of 39.19% (n=29) followed by roots and seeds which represented 27% (n=20) and 10.81% (n=8), respectively (Figure 2). Leaves were the most commonly used plant parts by the traditional healers in the herbal preparations which represented 39.19%. This could be due to ease of preparation of medicinal remedies13 and the presence of more biologically active components in leaves developed in response to phytophagous organisms.14
Figure 2.
The medicinal plant parts used widely to treat human and livestock ailments.
Methods of Preparation, Route of Administration and Application
The traditional practitioners used different methods of preparation and administration for the treatment of different types of human and livestock ailments. The preparation and administration systems differ based on the type of condition treated and the location of the disease. In this study, oral route of administration is the most commonly used route of application of the medicinally important plant parts in the study area which consisted of 47 herbal remedies and this was followed by topical and nasal routes of administration which represented 14 and 4 herbal therapies, respectively (Table 3). During group discussion sessions, most informants reported that they preserved the plant material that they could not find in the dry or rainy season in various ways like pounding and saving the powder or hanging the intact plant material in the kitchen. Traditional healers reported processing remedies mainly through crushing (46.91%), powdering (18.52%), pounding (11.11%), and pressing (9.88%) (Table 4). Pounding and powdering are effective for the complete extraction of the potential content of the plant and increase the curative power of the medicine or its efficacy, as both increase the healing power of the remedy through faster physiological reaction.
Table 3.
Uses, Preparation Method and Route of Administration of Medicinal Plants Commonly Used in the Study Area
| Scientific Name | Preparation Method | Uses | Human/Livestock | Route of Application |
|---|---|---|---|---|
| Asystasia guttata (Forssk.) Brummitt | Fresh or dry root of this plant is crushed and homogenized in water and the patient drinks it | Diarrhea | Human | Oral |
| Barleria argentea Balf. f. | Fruit is crushed and mixed with water and the patient drinks it | Diarrhea | Human | Oral |
| Barleria eranthemoides R. | Root is crushed, boiled and the patient drinks it | Stabbing pain | Human | Oral |
| Allium sativum L. | Bulb of this plant is powdered and consumed with honey | Malaria | Human | Oral |
| Aloe monticola Reynolds | Root is powdered and mixed with water and the patient drinks it | Anthrax | Human | Oral |
| Aloe macrocarpa Tod | Leaf of this plant is milled and mixed with honey and consumed by the patient | Wart | Human | Oral |
| Amaranthus caudatus L. | Leaf is crushed and boiled with water and drunk by the patient | Diarrhea | Human | Oral |
| Achyranthes aspera L. | Leaf is crushed, boiled and drunk by the patient | Itching sore | Human | Oral |
| Carissa edulis Vahl | Roots are boiled with water. A concentrated substance of the roots is used to treat malaria | Malaria | Human | Oral |
| Carissa spinarum L. | Fresh root of this plant is powdered and mixed with local alcohol and the patient drinks it | Impotence Stomach-ache Gonorrhea, Headache |
Human | Oral |
| Acmella caulirhiza Del. | Flower of this plant is chewed and spitted for tonsillitis | Tonsillitis | Human | Sublingual |
| Artemisia abyssinica Sch.Bip.ex.Rich/ | Fresh root of this plant is milled and mixed with water and the patient smells and drinks it | Evil spirit | Human | Oral+Nasal |
| Echinops kebericho, Mesfin | Dry root of this plant is crushed and mixed with coffee and the patient drinks and brushes teeth with it | Toothache Vomiting Headache |
Human | Oral+Sublingual |
| Guizotia scabra (Vis.) Chiov. | Leaf of this plant is pressed and drops are prepared and applied on the wound | Wound | Human | Topical |
| Balanites aegyptica (L.) Del. | Fresh or dry root of this plant is crushed and solubilized in water and the patient smells and drinks it | Evil spirit | Human | Oral+Nasal |
|
Cynoglossum amplifolium Hochst. ex DC. |
Fresh leaf of this plant is crushed and mixed with water and the patient smells it | General malaise | Human | Nasal |
| Coronopus didymus (L.) Sm | Leafy-stem of this plant is dried in sunlight, crushed and mixed with ingredient and the patient drinks it | Bone fracture | Human | Oral |
| Opuntia ficus-indica (L.) Mill. | Leaf of this plant is crushed and smoked in places where mosquitoes are present | Insecticidal | Human | Smoking |
| Crateva adansonii D.C. Prodr | Dry root of this plant is milled and solubilized with water and the patient drinks it | Gonorrhea | Human | Oral |
| Ritchiea albersii Gilg | The seed of this plant is powdered and mixed with hot water and drunk | Cough | Human | Oral |
| Cadaba farinose Forssk. | Young leaves are crushed and the juice drunk to treat malaria | Malaria | Human | Oral |
| Catha edulis (Vahl) | Fresh leaf is crushed and boiled with water and the patient drinks it | Gonorrhea | Human | Oral |
| Chenopodium ambroisoides L. | Dry leaf or stem of this plant is crushed, mixed with water and creamed on the hair | Dandruff | Human | Topical |
| Combertum paniculatum Schinz | Bark latex of this plant is powdered and mixed with table salt and creamed on affected skin | Ringworm | Human | Topical |
| Kalanchoe densiflora Rolfe | Fresh leaves of this plant are pressed, and drops are applied on the wound | Gonorrhea | Human | Topical |
| Coccinia grandis (L.) Voigt | Fresh or dry root of this plant is crushed and homogenized with water and applied to the infected site | Kidney infection | Human | Topical |
|
Cucumis dipsaceus Ehrenb. ex Spach |
Fresh fruit of this plant is crushed and extracted and drunk by the patient | Meningitis Hemorrhoids Rabies |
Human | Oral |
| Cucurbita pepo L. | Seed powder is mixed with water and filtered and the patient drinks it. Fresh leaves of this plant are crushed and homogenized with water and drunk by cattle |
Gonorrhea Epilepsy in cattle |
Human Livestock |
Topical Oral |
|
Dioscorea quartiniana A. Rich. |
Fresh or dry root of this plant is crushed and homogenized with water and the patient drinks it. Fresh root of this plant is crushed and boiled with water and the cattle drink it |
Vomit & diarrhea Kidney infection Side pain |
Human Livestock |
Oral Oral |
| Sansevieria ehrenbergii Schweinf. ex Bak. | Fresh or dry root or bark of this plant is crushed and tied on the swelling part | Swelling | Human | Topical |
| Croton macrostachyus Del. | Leafy-stem of this plant is crushed, mixed with water and butter and applied to the infected area and wound. The powder is mixed with water and the patient drinks it for the treatment of malaria |
Wound Gonorrhea Malaria |
Livestock Human Human |
Topical Oral |
|
Euphorbia abbyssinica J.F. Gmel. |
Fresh bark of this plant is concentrated after being boiled with water and the patient drinks it | Gastrointestinal, Gonorrhea |
Human | Oral |
| Euphorbia dumalis S. Carter | Fresh root of this plant is powdered and mixed with water and the patient drinks it | Liver case | Human | Oral |
| Euphorbia heterophylla L. | Fresh stem of this plant is crushed and extracted, part is applied on the hair | Dandruff | Human | Topical |
|
Justicia schimperiana (Nees) T. Anderson |
Dry or fresh seed of this plant is crushed and mixed with water and taken by the patient | Rabies | Human | Oral |
| Acacia abyssinica Hochst ex.Benth. | Fresh leaf of this plant is crushed and the juice is made by mixing with honey or sugar and the patient drinks it | Goitre | Human | Oral |
| Acacia hockii De Wild | The leaves are crushed and mixed with water and drunk by animals | Livestock medicines | Livestock | Oral |
|
Acacia mellifera (Vahl) Benth. |
Fresh leaf of this plant is powdered and dissolved with ingredients and then applied to the infected area | Body infection | Human | Topical |
| Albizia anthelmintica Brongn. | Dry bark of this plant is pounded and mixed with honey and coffee and eaten by the patients | Tape worm | Human | Oral |
| Albizia schimperiana Oliv. | Fresh root of this herb is dried, pounded and put on a fire and the smoke is used by the patient. Root of A. schimperiana is powdered and the powder is rolled in clean cloth and tied to the neck of equines. |
Evil eye Swelling |
Human | Nasal |
| Caesalpinia volkensii Harms | Dry seed of this plant is crushed and dissolved with water and the patient drinks it | Infertility problem in females | Human | Oral |
|
Calpurnia subdecandra (L’Herit.) Schweick. |
Fresh leaf of this vegetable is crushed and applied on the inflamed area | Skin diseases | Human | Topical |
| Erythrina abyssinica Lam. Ex. DC. | Fresh bark of this herb is powdered and mixed in water and the patient drinks it | Abdominal distension, and cramp | Human | Oral |
| Indigofera dauensis Gillett | Dry or fresh root of this shrub is crushed and tied on the body of the patient | Evil eye | Human | Topical |
| Pterolobium stellatum (Forssk.) | Fresh leaf of this plant is crushed and the patient drinks it with water. Fresh or dry root of this plant is crushed and boiled with water and the patient drinks it |
Sore throat, Sharp pain | Human | Oral Oral |
| Taverniera abyssinica A. Rich, | Dry root of this plant is smoked close to the patient | Spiritual disease | Human | Nasal |
| Ajuga integrifolia Buch.-Hamn. | Dry leaf of this herb is milled and mixed with fat and the patient drinks it | Internal Parasite Epilepsy |
Human | Oral |
| Clerdendrum myricoides Hochst | Fresh leaf of this vegetable is extracted by the process of maceration and the patient drinks it | Constipation General malaise |
Human | Oral |
| Ocimum gratissimum L. | Fresh leaf of this herbal is pressed and drops are applied to the inflamed area | Allergic | Human | Topical |
| Ocimium lamifolium Hochst. Ex. Benth. | Fresh leaf of this plant is crushed and smelled | Headache | Human | Nasal |
| Cassytha filliformis L. | Fresh or dry root of this plant is crushed and the patient inhales and drinks with water | Evil eye | Human | Nasal+ oral |
| Buddleja polystachya Fresen. | Fresh leaf of this shrub is squashed and spat on cattle’s eyes | Eye disease | Livestock | Topical |
| Bersama abyssinica Fresen. | Fresh leafy-stem of this plant is pressed and applied on the affected area | Wound | Human | Topical |
| Ficus sycomorus L. | Fluid is collected from bark surface of this plant and applied on inflamed area | Hepatitis | Human | Topical |
| Glinus lotoides L. | Leafy-stem of this herb is powdered and their juice is prepared by mixing with sweetening agent and the patient drinks it | Tape worm | Human | Oral |
| Maisa lanceolata Forssk. | Fresh or dry bark of this plant is milled and mixed with fat and applied to the affected area | Elephantiasis | Livestock | Oral |
| Eucalyptus globules labing | Fresh leaf of this plant is crushed and boiled in water and the patient drinks it | Influenza Allergic |
Human | Oral |
|
Cynodon dactylon L. Pers |
Fresh leafy-stem is collected and given to cattle | Bone fracture | Livestock | Oral |
| Cynodon nlemfuensis Vanderyst | Fresh leaf stem is crushed by teeth | Tonsillitis | Human | Oral |
| Phytolocca dodecandra L ‘Hert. | Fresh leaf is pressed and juice is prepared and the patient drinks it | Sinus Anemia |
Human | Oral |
| Plumbago zeylanica L. | Fresh leaf is pressed and juice is prepared and the patient drinks it | Cancer | Human | Oral |
| Rumex neppalensis Spreng | Dry or fresh root is powdered and mixed with coffee and the patient drinks it | Stomach pain | Human | Oral |
| Securidica longipedunculata Fresen | Dry and fresh root is milled and homogenized with water and the patient drinks it | Intestinal parasite | Human | Oral |
| Nigella sativa L. | Dry seed of this plant is powdered and mixed with water and the patient drinks it | Headache | Human | Oral |
| Prunus africana (Hook. f.) Kalkman | Fresh bark of this plant is extracted with water and juice is made and the patient drinks it | Prostate gland hypertrophy | Human | Oral |
| Coffee arabica L. | The seed of coffee is roasted and milled and mixed with honey and consumed by the patient | Diarrhea | Human | Oral |
| Clausena anisata (Wild.) Benth. | Fresh leaf of this plant is crushed and applied to the irritated area | Skin irritation | Human | Topical |
| Ruta chalepensis L. | Fresh leaf of this plant is crushed and drunk by the patient with alcohol | Stomach-ache | Human | Oral |
|
Brucea antidysentrica J.F.Mill. |
Fresh leaf of this plant is powdered and homogenized with water | Parasite | Human | Topical |
| Datura stramonium L. | Fresh leafy-stem is pressed and droplets are prepared with fat | Wart, Toothache | Human | Sublingual |
| Withania somnifera L. Dunal | Fresh of leaf is pounded, and juice is prepared and the patient drinks it | Malaria | Human | Oral |
| Zingiber officinale Roscoe | Fresh leafy-stem of this herb is powdered and mixed with gargle and other ingredients and drunk by the patient | Influenza, Internal Parasite | Human | Oral |
Table 4.
Mode of Preparation of Medicinal Plants
| Methods of Preparation | Frequency | Percentage (%) |
|---|---|---|
| Pounding | 9 | 11.11 |
| Crushing | 38 | 46.91 |
| Powdering | 15 | 18.52 |
| Chewing | 2 | 2.47 |
| Burning and inhaling | 2 | 2.47 |
| Extraction | 5 | 6.17 |
| Boiling | 2 | 2.47 |
According to the discussions made with traditional healers the preparations made were drawn from mixtures of different plant species with different additive substances such as honey, sugar, teff flour, butter, soda ash, salt, ground honey, soil, and charcoal ash for the treatment of single ailment (data not given). These additive substances had been reported to have a double function i.e. to improve flavour and reduce adverse effects such as vomiting and diarrhea, and enhance the efficacy and healing conditions.
In addition to the medicinal plants commonly used by the traditional healers for the treatment of different diseases, there were also reports of different diseases that the medicinal plants were prescribed for. The most common disease in which the herbal preparations were prescribed for were gonorrhea in which a total of six different medicinal remedies were prescribed and treated by the traditional healers. This was followed by remedies for diarrhoea and evil spirit which consisted of five medicinal plants reported as effective means of treatment of these cases. Also, four medicinal floras were reported as effective means of treatment of malaria.
Preference Ranking
The degree of agreement on ranking of 10 medicinal plants based on the principle that the plant species are widely known and frequently used for the treatment of a particular ailment among the local community is indicated in Table 5. Barleria argentea Balf. f. scored the highest points and ranked first followed by Dioscorea quartiniana A.Rich. and Euphorbia abbyssinica J.F. Gmel. species as widely known by the large local community and even prepared and used at family level.
Table 5.
Ranking of 10 Medicinal Plants Known in the Study Area by the Traditional Healers as Responded by 10 Informants
| Species Scientific name(s) | *R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | Total | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barleria argentea Balf. f. | 10 | 9 | 8 | 8 | 9 | 9 | 10 | 9 | 9 | 10 | 108 | 1 |
| Dioscorea quartiniana A.Rich. | 9 | 8 | 10 | 8 | 7 | 7 | 9 | 7 | 9 | 8 | 96 | 2 |
| Croton macrostachyus Del. | 5 | 6 | 7 | 5 | 8 | 6 | 6 | 7 | 6 | 7 | 75 | 5 |
| Phytolocca dodecandra L’Hert | 4 | 5 | 6 | 7 | 5 | 4 | 3 | 5 | 4 | 3 | 57 | 9 |
| Euphorbia abyssinica J.F. Gmel. | 8 | 7 | 6 | 8 | 6 | 8 | 1 | 6 | 8 | 7 | 89 | 3 |
| Cadaba farinose Forssk. | 5 | 6 | 4 | 7 | 6 | 6 | 7 | 5 | 5 | 7 | 69 | 6 |
| Coronopus didymus (L.) Sm | 4 | 5 | 5 | 6 | 7 | 4 | 5 | 6 | 7 | 4 | 62 | 8 |
| Ocimum gratissimum L. | 6 | 7 | 5 | 5 | 6 | 4 | 6 | 5 | 5 | 5 | 65 | 7 |
| Glinus lotoides L. | 4 | 6 | 7 | 7 | 4 | 8 | 3 | 2 | 1 | 3 | 56 | 10 |
| Crateva adansonii D.C. Prodr | 6 | 7 | 7 | 5 | 8 | 7 | 6 | 6 | 5 | 7 | 76 | 4 |
| *R = Respondent. |
Traditional Healers’ Consensus on Treating Different Human Ailments
High degree of consensus (ICF = 0.87) was observed among the traditional healers in treating malaria; this disease was treated by employing Allium sativum L., Carissa spinarum L., Cadaba farinose Forssk., Croton macrostachyus Del., and Withania somnifera (L.) Dun. (Table 6). Traditional healers agreed more in the treatment of diarrhea (ICF = 0.78), gonorrhea (ICF = 0.65) and evil spirit (ICF = 0.44). The species with the highest level of fidelity (FL = 100%) is in treatment of malaria. The highest fidelity level was recorded on the use of Carissa spinarum L., Cadaba farinose Forssk., Coffea arabica L., Dioscorea quartiniana A.Rich., Catha edulis (Vahl), Cucurbita pepo L., Artemisia abyssinica, Taverniera abyssinica A. Rich, by traditional healers of the study area to treat malaria, diarrhea, gonorrhea and evil spirit, respectively.
Table 6.
Healers’ Consensus Factor and Fidelity Levels
| Ailments | ICF | Species | FL |
|---|---|---|---|
| Gonorrhea | 0.65 | Carissa spinarum L. | 36.5 |
| Crateva adansonii D.C. Prodr | 48.7 | ||
| Catha edulis (Vahl) | 74.3 | ||
| Kalanchoe densiflora Rolfe | 21.9 | ||
| Cucurbita pepo L. | 53.6 | ||
| Croton macrostachyus Del. | 19.5 | ||
| Euphorbia abyssinica J.F. Gmel. | 46.3 | ||
| Diarrhoea | 0.78 | Asystasia guttata (Forssk.) Brummitt | 74.3 |
| Barleria argentea Balf. f. | 40.2 | ||
| Amaranthus caudatus L. | 65.8 | ||
| Dioscorea quartiniana A.Rich. | 80.5 | ||
| Coffea arabica L. | 86.6 | ||
| Evil spirit | 0.44 | Artemisia abyssinica Sch.Bip.ex.Rich/ | 76.8 |
| Balanites aegyptica (L.) Del. | 28.1 | ||
| Albizia schimperiana Oliv. | 65.85 | ||
| Indigofera dauensis Gillett | 30.5 | ||
| Taverniera abyssinica A. Rich, | 71.9 | ||
| Malaria | 0.87 | Allium sativum L. | 78.1 |
| Carissa spinarum L. | 100 | ||
| Cadaba farinose Forssk. | 90.2 | ||
| Croton macrostachyus Del. | 75.6 | ||
| Withania somnifera (L.) Dun. | 19.5 |
Physicochemical Analysis
Based on the degree of agreement on ranking of 10 medicinal plants and the principle that the plant species are widely known and frequently used for the treatment of a particular ailment among the local community, the top six medicinal plants were selected for further processing for physicochemical investigation (Table 7). The plant parts were dried, powdered, and passed through a sieve and appropriate amounts of the sample were taken to undergo analysis.
Table 7.
Selected Medicinal Plants for Further Processing for Physicochemical Investigation
| S. No. | Local Name | Plant Name | Parts Used | Disease(s) Treated |
|---|---|---|---|---|
| 1 | Hokto | Barleria argentea Balf. f. | Fruit | Diarrhea |
| 2 | Gishu | Dioscorea quartiniana A.Rich. | Fresh or dry root | Diarrhea |
| 3 | Adaamii | Euphorbia abyssinica J.F. Gmel. | Bark | Gonorrhea |
| 4 | Qolladii | Crateva adansonii D.C. Prodr | Dried root | Gonorrhea |
| 5 | Bakkannisa | Croton macrostachyus Del. | Powdered leafy-stem | Malaria |
| 6 | Galee | Cadaba farinose Forssk. | Young leaves | Malaria |
Determination of Extractable Matter
The average concentration of water-soluble extractive value and alcohol-soluble extractive value of the extracted medicinal plants included in this study is summarized in Table 8. Accordingly, water-soluble extractive value of Euphorbia abbyssinica J.F. Gmel. was higher than the rest at 18.53%w/w and followed by Dioscorea quartiniana A.Rich. and Barleria argentea Balf. f. which represented 18.507 and 16.217%w/w average concentration of water-soluble extractive value, respectively. A lower average concentration of water-soluble extractive value was detected in Cadaba farinose Forssk. (1.825%w/w). These results show the presence of water-soluble additives like sugar, acids, and inorganic compounds, etc. The average concentration of alcohol-soluble extractive value of Cadaba farinose Forssk. was high at 1.107%w/w and followed by Crateva adansonii D.C. Prodr and Dioscorea quartiniana A.Rich. with 0.33 and 0.329%w/w, respectively. These indicates the presence of contents such as phenols, alkaloids, steroids, glycosides, and flavonoids. Comparing the water-soluble and alcohol-soluble extractive values of the medicinal plants, it was concluded that the percent water-soluble extractive values were higher than the alcohol-soluble extractive values; these indicates presence of more amounts of water-soluble contents in the plants.
Table 8.
Extractive Matters and Moisture Contents of the Selected Medicinal Plants in Southwest Ethiopia (Mean (%w/w) ± SD)
| S. No | Plant Name | Water Extractable Values (n=3) | Alcohol Extractable Values (n=3) | Moisture Contents (n=3) |
|---|---|---|---|---|
| 1 | Barleria argentea Balf. f. | 16.217±4.018 | 0.166±0.116 | 21.063±0.988 |
| 2 | Dioscorea quartiniana A.Rich. | 18.507±3.475 | 0.329±0.215 | 15.397±5.716 |
| 3 | Euphorbia abyssinica J.F. Gmel. | 18.53±1.19 | 0.143±0.067 | 16.347±6.829 |
| 4 | Crateva adansonii D.C. Prodr | 14.515±3.191 | 0.33±0.115 | 18.143±4.582 |
| 5 | Croton macrostachyus Del. | 15.185±2.09 | 0.271±0.0996 | 15.73±4.148 |
| 6 | Cadaba farinose Forssk. | 1.825±0.559 | 1.107±0.111 | 4.876±0.999 |
Determination of Moisture Contents
The results of the moisture content determinations are presented in Table 8. The moisture content (%LOD) of Barleria argentea Balf. f. was higher than the recommended standard at 21.063%w/w and followed by high %LOD of Crateva adansonii D.C. Prodr and Euphorbia abbyssinica J.F. Gmel. which represented 18.143 and16.347%w/w, respectively (Table 8). This shows that the drugs developed high moisture contents after being properly dried and stored. Only one medicinal plant has normal moisture content, this being Cadaba farinose Forssk. (4.876%w/w). Moisture content (%LOD) is mainly used to regulate the pleasantness of herbal preparations. The pharmacopoeial monographs limit the water content material in vegetable sources to between 8% and 14%.
Determination of Ash Contents
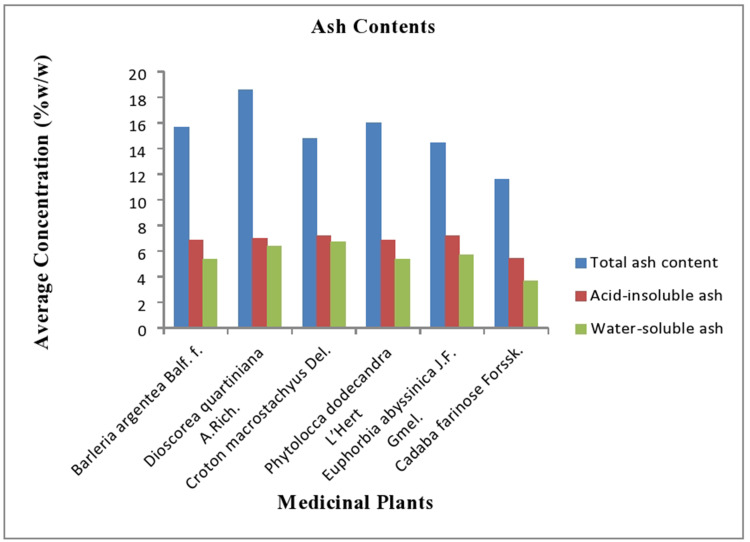
The purity of herbal extracts can also be assessed by determination of ash values which constitute the content of foreign matter together with inorganic salts or silica present as a shape of adulterant within the drug sample. In this study, the highest total ash value was registered in Dioscorea quartiniana A.Rich. species which consisted of 18.563%w/w and followed by Crateva adansonii D.C. Prodr and Barleria argentea Balf. f. with 16.033 and 15.648%w/w, respectively (Figure 3). This shows the presence of physiological ash that is derived from the plant tissue itself, and non-physiological ash.
Figure 3.
Total ash content, acid-insoluble ash, and water-soluble ash of the selected medicinal plants Southwest Ethiopia.
The acid-insoluble ash value of Euphorbia abbyssinica J.F. Gmel. was higher than the rest at 7.227%w/w and followed by the acid-insoluble ash value of Croton macrostachyus Del. and Dioscorea quartiniana A.Rich. with 7.217 and 6.975%w/w (n=3), respectively. This indicates the presence of sand and siliceous earth. The amount of water-soluble ash was approximately equal to the acid-insoluble ash value with the highest result frequently observed for Euphorbia abbyssinica J.F. Gmel. species with 6.731%w/w (n=3). The average concentration of water-soluble ash for Dioscorea quartiniana A.Rich. species was relatively equal with the value of Euphorbia abbyssinica J.F. Gmel. with 6.398%w/w (n=3). The results of acid-insoluble ash values and water-soluble ash values of the selected medicinal plants are summarized in Figure 3.
Discussion
This study revealed that 72 medicinal plants were stated by the respondents for the treatment of different human and livestock ailments and their specimens were collected from the traditional healers’ own gardens and from forests. The collected specimens were identified based on herbal standard preparation procedures and categorized under 61 genera and 39 families. Family Fabaceae was the largest medicinal plant family used for the treatment of different diseases in the study area, representing 11 species (15.28%). This finding was similar to the ethnobotanical studies reported from different areas of Ethiopia which also showed that family Fabaceae is the largest medicinal family mostly used by the local healers for healing common diseases.3,15,16 This could be attributed to their wider spreading, abundance, and rich bioactive constituents of medicinal plants in Ethiopia. Family Euphorbiaceae was the second most used group of medicinal plants by the community next to the Fabaceae, consisting of five species (6.94%), followed by Lamiaceae and Asteraceae which consisted of four species each (5.56%).
The medicinal plant parts used widely to treat human and livestock ailments include leaves, root, stem, and others. The most commonly used medicinal plant parts for the preparation of medicines into different dosage forms by the traditional healers in the study area were leaves which consisted of 39.19% followed by roots and seeds which represented 27% and 10.81%, respectively. This could be due to the ease of preparation of medicinal remedies from leaves13 and the presence of more biologically active components in leaves due to phytophagous organisms.14 This result is agree with the study reported in other parts of Ethiopia in which leaves were most commonly used to treat various human and livestock diseases.3,15,16
The use of more than one species was stated by the traditional practitioners to treat human and animal diseases, which could be attributed to the additive or synergistic effects of the mixtures.17 Similarly, a study in South Africa indicated that herbs and shrubs plant species were essential foundations for complementary and alternative medicines.18 Conversely, a study done in southern Ethiopia showed that 30% of documented medicinal floras were trees and in addition various authors reported that conventional medicine includes information of human and livestock ailment treatments.19
Traditional healers reported processing remedies mainly through crushing (46.91%), powdering (18.52%), pounding (11.11%), and pressing (9.88%). Pounding and powdering are effective for the complete extraction of the potential content of the plant and increase the curative power of the medicine or its efficacy, as both increase the healing power of the remedy through faster physiological reaction. The traditional practitioners used different methods of preparation and administration for the treatment of different types of human and livestock sicknesses. The preparation and administration systems differ based on the type of ailment treated and the location of the disease. In this study, oral route of administration is the most commonly used route of application of the medicinally important plant partswhich consisted of 47 herbal remedies and this was followed by topical nasal routes of administration which represented 14 and 4 herbal therapies, respectively. Similar results were reported in other parts of Ethiopia in which oral route of administration is the most commonly used route for application of herbal preparations by the traditional practitioners.3,16
In addition to the medicinal plants commonly used by the traditional healers for the treatment of different diseases, there were also report of different diseases that the medicinal plants were prescribed for. The most common disease in which the herbal preparations were prescribed for were gonorrhea in which a total of six different medicinal remedies were prescribed and reported by the tradition healers. Similar results were reported from the study done on ethnobotany of medicinal plants in southeastern Ethiopia in which the major and most widespread diseases according to the informants include gonorrhea, jaundice, kidney infection, and general malaise.20 These are followed by diarrhea and evil spirit for which five medicinal plants were reported as effective means of treatment. Also, four medicinal flora were reported as effective means of treatment of malaria.
Quality management of herbal extracts/preparations relates to the physicochemical assessment of crude drugs covering the aspects such as choice and dealing with the crude material, safety, efficacy and balanced evaluation of completed product.21 Physicochemical standards had been usually used for assessing the identity, purity, and strength of the drug supply. These parameters were also used to identify any adulterants if existing in the plant substance.22
In this study, water-soluble extractive value of Euphorbia abbyssinica J.F. Gmel. was higher than the rest at 18.53%w/w and followed by Dioscorea quartiniana A.Rich. and Barleria argentea Balf. f. with 18.507 and 16.217%w/w of average concentration of water-soluble extractive value, respectively. A low average concentration of water-soluble extractive value was detected in Cadaba farinose Forssk. (1.825%w/w). These results showthe presence of water-soluble additives such as sugar, acids, and inorganic compounds, etc. A lower extractive value indicates addition of exhausted material, adulteration or incorrect processing during drying, storage or formulation. The average concentration of alcohol-soluble extractive value of Cadaba farinose Forssk. was high at 1.107%w/w and was followed by Crateva adansonii D.C. Prodr and Dioscorea quartiniana A.Rich. at 0.33 and 0.329%w/w, respectively. This indicates the presence of contents such as phenols, alkaloids, steroids, glycosides, and flavonoids. This result is relatively similar with the study conducted on the leaves of Carallia brachiate in India which showed that their water-soluble extractive value of the leaves was 11.63 ±0.60 (%w/w) and a higher alcohol-soluble extractive value was reported (3.96 ±0.12%w/w) compared with the present study.23
In this study, the moisture content (%LOD) of Barleria argentea Balf. f. was higher than the recommended standard at 21.063%w/w and followed by high %LOD of Crateva adansonii D.C. Prodr and Euphorbia abbyssinica J.F. Gmel. with 18.143 and 16.347%w/w, respectively. This shows that the drug absorbed moisture after being properly dried and stored. Only one medicinal plant had normal moisture content, this being Cadaba farinose Forssk. (4.876%w/w). Moisture content (%LOD) is mainly used to regulate the pleasantness of herbal preparations. The pharmacopoeial monographs limit the water content material in vegetable sources at 8% and 14%.
High moisture content corresponds frequently to an excessive amount of free water which could alter various physicochemical properties of merchandise which is intended for use or stored dry; it also assists the growth of dangerous bacteria, yeasts, and molds.24 The European Agency for the evaluation of medicinal products recommended that the moisture content must be included within the listing of comprehensive specifications for herbal materials and finished natural merchandise which can be solids, since the influence of water content on the stability and safety of crude drugs is significant.25 To manipulate moisture in herbal merchandise, the USP prescribes storage under a relative humidity of below 60% RH.
The purity of herbal extracts can also be assessed by determination of ash values which constitute the content of foreign matter together with inorganic salts or silica present as adulterants within the drug sample. In this study, the highest total ash value was registered in Dioscorea quartiniana A.Rich. species at 18.563%w/w and followed by Crateva adansonii D.C. Prodr and Barleria argentea Balf. f. at 16.033 and 15.648%w/w, respectively. This shows the presence of physiological ash that is derived from the plant tissue itself, and non-physiological ash. This is similar with a study conducted in India that documented the total ash to be 17.83±0.20%w/w.26
In this study, the acid-insoluble ash value of Euphorbia abbyssinica J.F. Gmel. was higher than the rest at 7.227%w/w and followed by the acid-insoluble ash value of Croton macrostachyus Del. and Dioscorea quartiniana A.Rich. at 7.217 and 6.975%w/w (n=3), respectively. This indicates the presence of sand and siliceous earth. The amount of water-soluble ash was approximately equal with the acid-insoluble ash value with the highest result frequently observed for Euphorbia abbyssinica J.F. Gmel. species at 6.731%w/w (n=3); the average concentration of water-soluble ash for Dioscorea quartiniana A.Rich. species was relatively equal to the value of Euphorbia abbyssinica J.F. Gmel. at 6.398%w/w (n=3). The ash content gives an idea of the inorganic content of powdered leaves under investigation and thus the standard of the drugs is often assessed. This result is comparably similar with a study from India which showed that the amounts of acid-insoluble and water-soluble ash were 8.26±0.23 and 7.3±0.28 (%w/w), respectively.26
Conclusions
This finding reveals that there is a rich indigenous knowledge of medicinal plant use in the study area. Leaves were the most commonly used plant parts by the traditional healers in the herbal preparations. High degree of consensus was observed among the traditional healers in treating malaria. The most common diseases for which the herbal preparations were prescribed were gonorrhea, diarrhea, evil spirit, and malaria. The moisture content of all the medicinal plants studied was high enough to potentially compromise stability. The study also revealed that the water-soluble extractive value of the selected medicinal plants indicates the presence of water-soluble components such as sugar, acids and inorganic compounds, etc. and also the alcohol-soluble extractive value indicates the presence of constituents such as phenols, alkaloids, steroids, glycosides, and flavonoids. In the future, these characteristics can be used to check the genuine nature of the crude drug, thus these results play an important role in preventing the possible steps of adulteration and can also be used in the standardization of medicinal plants/crude drugs. It is essential to establish nationally recognized guidelines for assessing their quality and establishing monographs for herbal products.
Acknowledgments
The authors are grateful to Jimma University for allowing the analysis to be carried out in the Laboratory, and all the study participants.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
LOD, Loss on Drying; TS, Test Solution; WHO, World Health Organization.
Data Sharing Statement
All data generated or analyzed during this study are included in this manuscript. However, raw data are available from the corresponding author.
Ethics Approval and Consent to Participate
Verbal informed consent was approved by the Ethical Review Board of Jimma University.
This research was approved by the Ethical Review Board of Jimma University, Health research and post graduate director. Before starting the questionnaire, all participants who ultimately completed the questionnaire were informed of the purpose, content and other details of our study. Verbal informed consent was obtained from Traditional Healers prior to the interview. Confidentiality and privacy of the information was assured and maintained.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interests.
References
- 1.Kumar S, Kumar R, Khan A. Medicinal plant resources: manifestation and prospects of life-sustaining healthcare. Cont J Biol Sci. 2011;4(1):19–29. [Google Scholar]
- 2.Abramov V. Traditional medicine. World Health Org. 1996;134:1–3. [Google Scholar]
- 3.Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):40. doi: 10.1186/1746-4269-10-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okigbo R, Mmeka E. An appraisal of phytomedicine in Africa. KMITL. Sci Technol J. 2006;6(2):83–94. [Google Scholar]
- 5.Chauhan B, Kumar G, Kalam N, Ansari SH. Current concepts and prospects of herbal nutraceutical: a review. J Adv Pharm Technol Res. 2013;4(1):4–8. doi: 10.4103/2231-4040.107494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abebe W. Herbal medication: potential for adverse interactions with analgesic drugs. J Clin Pharm Ther. 2002;27(6):391–401. doi: 10.1046/j.1365-2710.2002.00444.x [DOI] [PubMed] [Google Scholar]
- 7.Firenzuoli F, Gori L, Galapai C. Adverse reaction to an adrenergic herbal extracts (Citrus aurantium). Phytomed. 2005;12(3):247–248. doi: 10.1016/j.phymed.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 8.Annan K, Dickson RA, Amponsah IK, Jato J, Nooni IK. Pharmacognostic evaluation and physicochemical analysis of Paullinia pinnata L. (Sapindaceae). J Pharmacogn Phytochem. 2013;2(2):203–208. [Google Scholar]
- 9.Belew S, Suleman S, Mohammed T, et al. Quality of fixed dose artemether/lumefantrine products in Jimma Zone, Ethiopia. Malar J 2019;18:236. doi: 10.1186/s12936-019-2872-1 [DOI] [PMC free article] [PubMed]
- 10.Abubakar IB, Ukwuani-kwaja AN, Sulaimon F, et al. An inventory of medicinal plants used for treatment of cancer in Kwara and Lagos state, Nigeria, Eur. J Integr Med. 2020;34:101062. doi: 10.1016/j.eujim.2020.101062 [DOI] [Google Scholar]
- 11.Ngoua-meye-misso R, Sima-obiang C, Ndong LJDC, et al. Medicinal plants used in management of cancer and other related diseases in Woleu-Ntem province, Gabon, Eur. J Integr Med. 2019;29:100924. doi: 10.1016/j.eujim.2019.05.010 [DOI] [Google Scholar]
- 12.World Health Organization (WHO). Quality control methods for herbal materials. World Health Organization; 2011. Available from: https://apps.who.int/iris/handle/10665/44479. Accessed February5, 2017. [Google Scholar]
- 13.Gazzaneo LRS, Lucena RFP, Albuquerque UP. Knowledge and use of medicinal plants by local specialists in a region of Atlantic forest in the state of Pernambuco (Northeastern Brazil). J Ethnobiol Ethnomed. 2005;1(1):9. doi: 10.1186/1746-4269-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattarai S, Chaudhary RP, Taylor RS. Ethnomedicinal plants used by the people of Manang district, central Nepal. J Ethnobiol Ethnomed. 2006;2(1):41. doi: 10.1186/1746-4269-2-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2006;110(3):516–525. doi: 10.1016/j.jep.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 16.Assegid A, Tesfaye A. Ethnobotanical study of wild medicinal trees and shrubs in Benna Tsemay District, Southern Ethiopia. J Sci Dev. 2014;2(1):17–33. [Google Scholar]
- 17.Igoli JO, Tor-Anyiin TA, Usman SS, Oluma HOA, Igoli NP. Folk medicines of the lower Benue valley of Nigeria In: Singh VK, Govil JN, Hashmi S, Singh G, editors. Recent Progress in Medicinal Plants. USA: Sci. Tech. Pub; 2002:327–338. [Govil JN and Singh VK (Series Editors) Ethnomedicine and Pharmacognosy, vol 7.]. [Google Scholar]
- 18.Mahwasane ST, Middleton L, Boaduo N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa: South African. J Bot. 2013;88:69–75. doi: 10.1016/j.sajb.2013.05.004 [DOI] [Google Scholar]
- 19.Regassa R. Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. J Med Plant Res. 2013;7(9):517–535. doi: 10.5897/JMPR012.1126 [DOI] [Google Scholar]
- 20.Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. 2008;4(1):10. doi: 10.1186/1746-4269-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A. Critical issues in quality control of herbal products. Pharm Times. 2005;37(6):9–11. [Google Scholar]
- 22.Singh R, Ali A, Jeyabalan G, Kumar Y, Semwal A. Development of quality control parameters for the standardization of bark of Ficus arnottiana Miq. (M). J Acute Dis. 2013;218–221. [Google Scholar]
- 23.Junejo JA, Zaman K, Rudrapal M, Mondal P, Singh KD, Kumar V. Preliminary phytochemical and physicochemical evaluation of Carallia brachiata (Lour.) Merr. leaves. J App Pharm Sci. 2014;4(12):123–127. doi: 10.7324/JAPS.2014.41221 [DOI] [Google Scholar]
- 24.Saohin W, Boonchoong P, Iamlikitkuakoon S, Jamnoiprom I, Mungdee W. Effects of drying temperature and residual moisture content of Fa-Tha-Li (Andrographis paniculata (Burm.f.) Nees) crude powder for capsule preparation: Thai. J Pharm Sci. 2007;31:28–35. [Google Scholar]
- 25.Anjoo K. Analytical Evaluation of Herbal Drugs, Drug Discovery Research in Pharmacognosy. Vallisuta O, Olimat SM, editors. IntechOpen; 2012. doi: 10.5772/26109 [DOI] [Google Scholar]
- 26.Philip F, Chris C, John A, Isreal E. Survey on the pharmaceutical quality of herbal medicines sold in Nigeria. J App Pharm Sci. 2015;5(06):097–103. [Google Scholar]