Figure 5.
Clinical Benefits of Dual PD-1 and CTLA-4 Blockade Mediated by MGD019
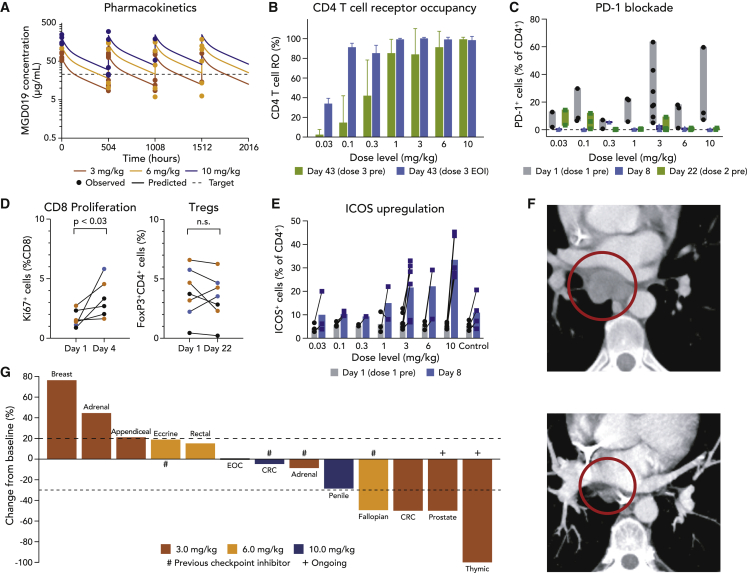
(A) Simulated multiple-dose PK profiles for the 3, 6, and 10 mg/kg Q3W regimens with observed pre-dose and post-dose data superimposed. Target concentration overlaid as dashed line.
(B) MGD019 receptor occupancy for CD4+ T cells collected 21 days after second infusion (green) compared to measured immediately after third infusion (blue) (N = 22). Means and SDs are depicted.
(C) Binding of MGD019-competing FACS mAbs to circulating T cells in patients treated with MGD019 before first dose (gray) and 8 (blue) and 22 (green) days later (N = 28). Bars indicate minimum to maximum intervals.
(D) Fraction of proliferating CD8+ T cells (N = 6) and regulatory T cells (N = 7) observed in cryopreserved PBMCs of patients treated with 3 (brown), 6 (yellow), and 10 (blue) mg/kg MGD019 collected at the indicated days. Paired t-test with two tailed p value calculation was used.
(E) ICOS expression on peripheral blood CD4+ T cells measured before (gray) and 8 days after (blue) first infusion of indicated doses of MGD019 (N = 28) or patients treated with a PD-1 based therapy not containing CTLA-4 blockade (obrtained from an independent study) that serves as a Control (N=4). Bars indicate mean values.
(F) Scans of the patient with microsatellite stable (MSS) colorectal cancer (CRC) obtained ~15 weeks after treatment initiation, demonstrating resolution of a 3.0-cm subcarinal lymph node.
(G) Waterfall plot of RECIST 1.1 response evaluable patients treated with 3, 6, and 10 mg/kg MGD019. # indicates previous treatment with checkpoint inhibitor and + indicates patients currently staying in the study.
See also Figure S6.