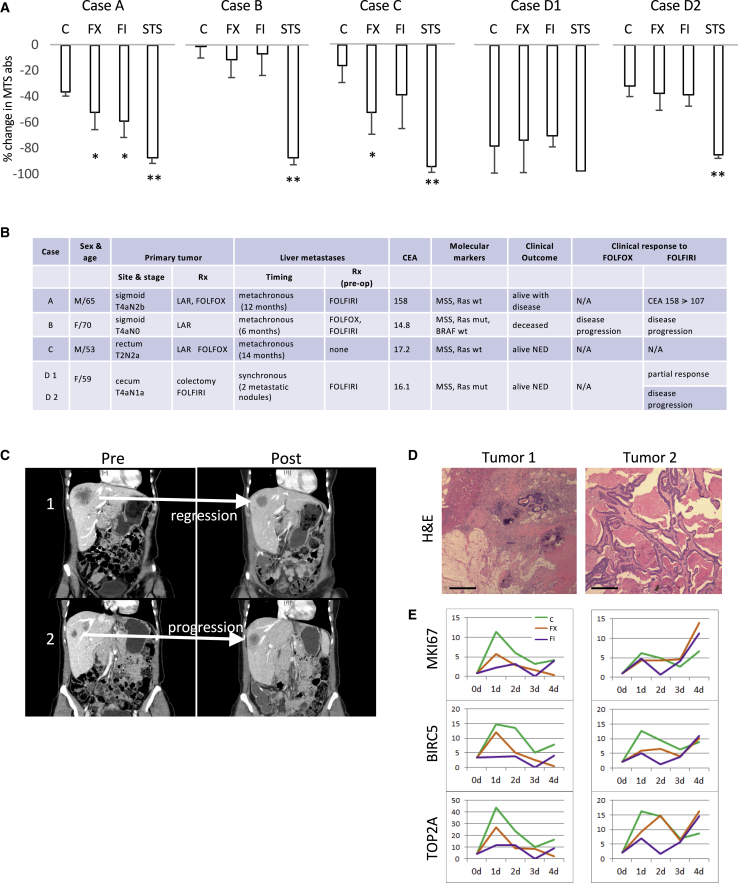
Figure 1.
Correlation between In Vitro and Clinical Responses
(A) Human CRLM slices from 5 tumors were treated with 5-FU/oxaliplatin (FX) and 5-FU/irinotecan (FI) for 72 h, and viability was assessed using an MTS assay. Results represent the percentage of change in MTS absorbance (mean ± SD) between time 0 and 72 h. A minimum of 3 tumor slices were used in each treatment. C, vehicle control; STS, staurosporine as positive control. ∗p < 0.05 and ∗∗p < 0.005 compared to control based on pairwise comparison (Student’s t test).
(B) Corresponding clinical characteristics of the cases shown in (A). CEA, carcinoembryonic antigen; LAR, low-anterior resection; mut, mutant; NED, no evidence of disease; wt, wild type.
(C) Coronal contrast CT images of case D showing divergent tumor response to FOLFIRI in 2 liver metastases. Tumor 1 responded to chemotherapy while tumor 2 progressed.
(D) H&E staining of the 2 tumors in case D. The blue (basophilic) cells highlight areas of viable tumors. Original magnification 100×. Scale bar, 200 μm.
(E) Temporal gene expression of 3 proliferation markers from bulk RNA-seq analyses of tumor slices derived from tumors D1 (left column) and D2 (right column) at 0, 24, 48, 72, and 96 h. The y axis represents fold change in transcript levels relative to day 0. Three tumor slices independent of the ones used in (A) from each time point were used for RNA extraction. BIRC5, baculoviral IAP repeat containing 5; MKI67, marker of proliferation Ki-67; TOP2A, DNA topoisomerase II alpha.