Figure 6.
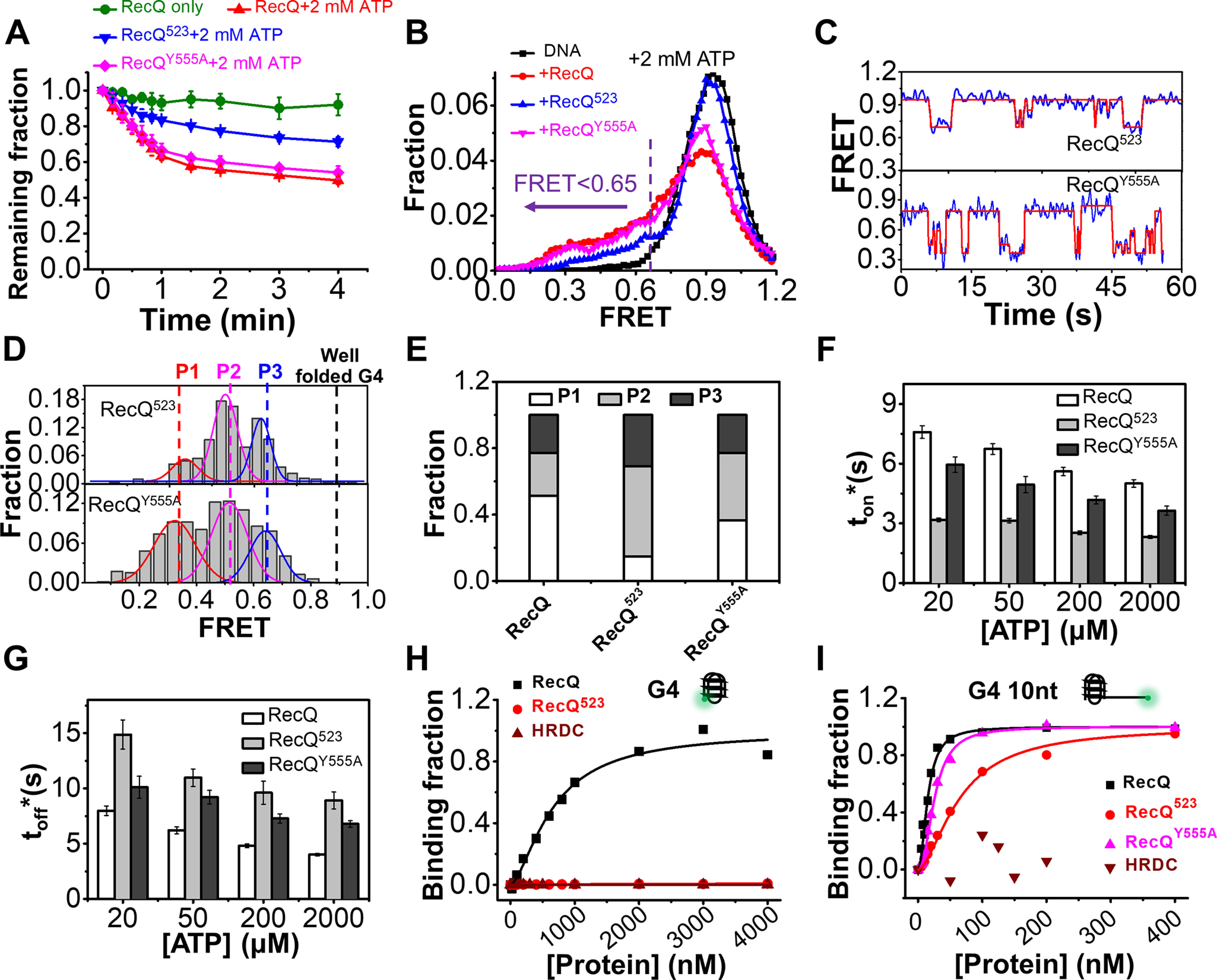
The HRDC domain of RecQ is necessary for the efficient unfolding of the G4 structure. A, fractions of remaining 29 bp-G4 12 nt molecules on coverslip versus time after the addition of 5 nm protein and 2 mm ATP. B, FRET distributions of 29 bp-G4 12 nt in 2 mm ATP and different RecQ constructs. According to the FRET peaks in Fig. 5F, a criterion at E0.65 was set artificially, below which the G4 structure was recognized as being disrupted. C, in 5 nm RecQ523 or RecQY555A and 20 µm ATP, the FRET values of 29 bp-G4 12 nt fluctuate between different levels. D, distributions of the FRET oscillation regions of the G4 substrate from ∼100 traces. E, the fractions of P1, P2, and P3 in the FRET histograms of G4 substrate in different types of RecQ. F and G, histograms of ton* and toff* in RecQ, RecQ523, and RecQY555A. H and I, RecQ constructs binding to G4 (H) or G4 10 nt (I) measured by equilibrium DNA binding assay. The dissociation constant (KD) of RecQ bound to G4 is 658.2 ± 79 nm; KD of RecQ523 and HRDC bound to G4 were both not available. KD of RecQ, RecQ523, and RecQY555A bound to G4 10 nt were 15.1 ± 1.1 nm, 63.7 ± 4.1 nm, and 25.6 ± 1 nm, respectively; KD of HRDC bound to G4 was not available.
