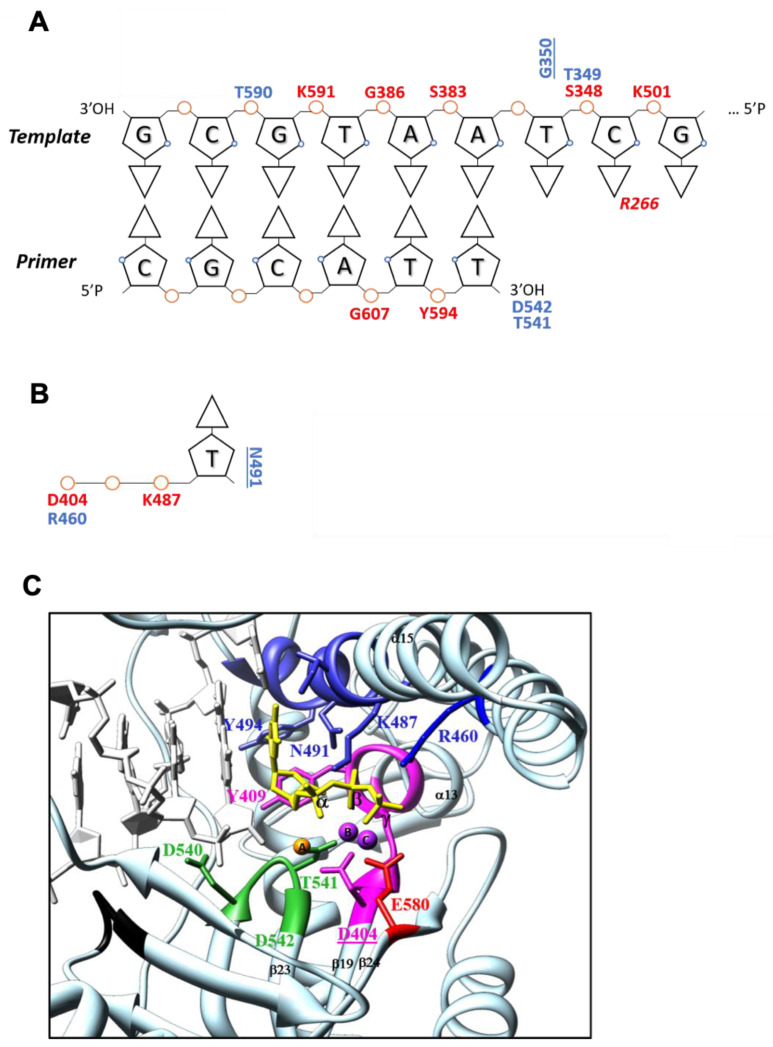
Figure 4.
TgoT_6G12 active site analysis. (A) Pattern of interactions between TgoT_6G12 polymerase and the template/primer DNA duplex, in the binary complex, according to the strength of the interactions. The color code for the strength of interactions is: red for strong/moderate and direct interactions and blue for weak electrostatic but direct interactions. Interactions between the enzyme and the phosphate backbone of DNA are labeled above, for the template, and below, for the primer, each phosphate (represented by an orange circle). Interactions between the enzyme and the ribose moieties are labeled, underlined and vertical. Interactions between the polymerase and the nucleotide base are in italics. (B) Interactions of TgoT_6G12 with the ddTTP incoming nucleotide using the same labeling scheme. (C) TgoT_6G12 active site with the incoming ddTTP (colored in yellow). Representative sequence motifs are labeled. The Kx3NSxYGx2G B-motif in the finger domain is colored in dark blue. The A-motif (DxxSLYPSI) is colored in magenta and the catalytic residue D404 is underlined. The C-motif (DTDG) of the palm domain is colored in green and the KKKY motif of the thumb domain is colored in black. One other residue of the palm domain, which interacts with the ddTTP, is colored in red. The ddTTP makes contacts with residues of the palm and finger subdomains (D404, K487, R460, and N491). The Mg2+ in position A is colored in orange. It is coordinated by residues D404 (A-Motif) and D542 (C-Motif). The two Mn2+ are colored in purple. The Mn2+ in position B is directly coordinated by residues D404 and D542 and by the three α,β,γ phosphates of the ddTTP. The Mn2+ in position C is coordinated by residues E580 and D404 and by the γ-phosphate of the ddTTP.