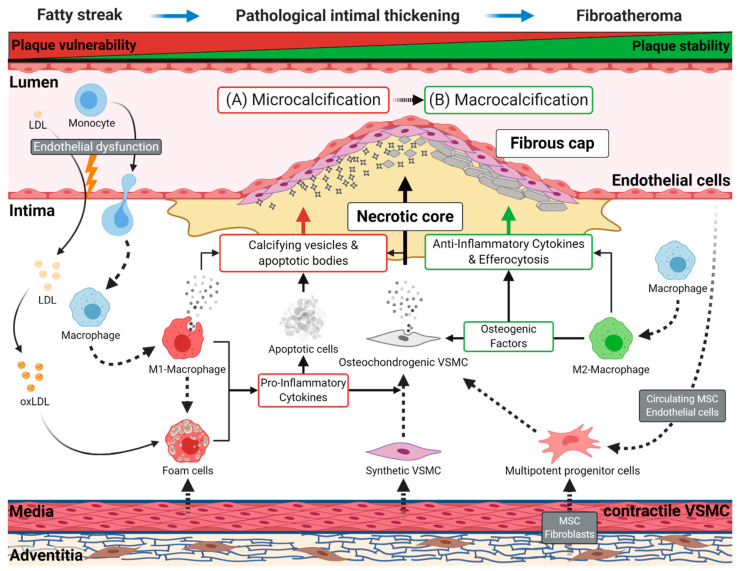
Figure 1.
Schematic representation of atherogenesis and intimal vascular calcification. Endothelial dysfunction promotes retention of low-density lipoprotein (LDL) in the intima and the diapedesis of monocytes. Subsequently, retained LDL transforms to oxidized LDL (oxLDL), and monocytes migrating to the intima differentiate into macrophages. Later, these cells can switch to pro-inflammatory M1 or anti-inflammatory M2 macrophages. Resting macrophages and M1 macrophages can absorb oxLDL, which converts them into foam cells. These cells start forming the lipid-rich, necrotic core of the atherosclerotic plaque, which first occurs as a fatty streak in the vessel wall, the earliest sign of atherogenesis. During disease progression, vascular smooth muscle cells (VSMC) acquire a synthetic phenotype and migrate into the plaque contributing to foam cell formation and the stabilization of the fibrous cap. Foam cells and M1 macrophages induce a chronic, pro-inflammatory environment through the secretion of numerous cytokines. The resulting chronical inflammation and the subsequent consumption of oxLDL leads to the apoptosis of these lipid-laden cells and others cell types, causing the shedding of apoptotic bodies into the atherosclerotic plaque. (A) During early atherogenesis (e.g., pathological intimal thickening, fibroatheromas), apoptotic bodies originating from apoptotic cells, and calcifying vesicles, secreted by M1 macrophages, serve as the first nidus for microcalcifications, favoring unstable plaque formation. Subsequently, contractile VSMCs obtain a proliferative synthetic phenotype, leading to their migration into the intima to build the fibrous cap. The continuous inflammatory environment, induced by M1 macrophages and foam cells, initiates the osteochondrogenic transition of synthetic VSMC, contributing to micro- and macrocalcification. (B) In the further course of atherogenesis, M2 macrophages promote the progression of calcification through extracellular matrix deposition and by secreting various osteogenic factors, which may favor the development of sheet-like calcifications. Subsequently, M2 macrophages resolve inflammation by secreting anti-inflammatory cytokines and by removing apoptotic cells through efferocytosis. These mechanisms lead to regression and stabilization of the atherosclerotic plaque. Other vascular cells, originating from the adventitia (fibroblasts, pericytes, mesenchymal stem cells) or the endothelial cell layer, are potential sources for multipotent progenitor cells. The progenitor cells may contribute to the osteochondrogenic cell population in the plaque.