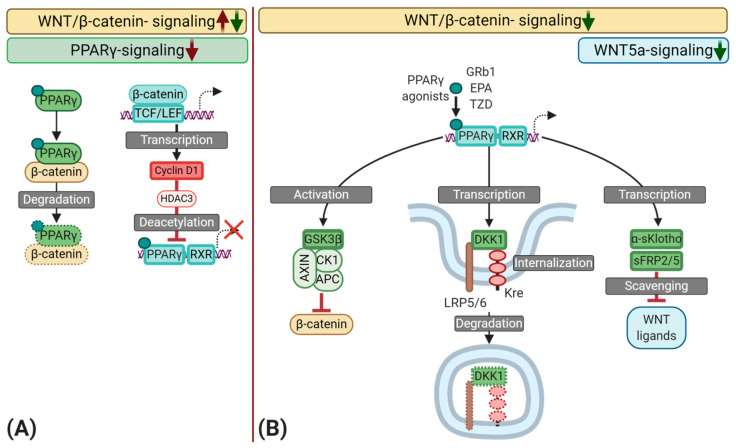
Figure 3.
Schematic representation of the interplay between peroxisome proliferator-activated receptor γ (PPARγ) and the Wingless and Int-1 (WNT) signaling pathways during vascular calcification (VC) with a focus on the osteochondrogenic transition of synthetic vascular smooth muscle cells (VSMC). (A) Several mechanisms may inhibit PPARγ signaling during atherogenesis. First, ligand-bound PPARγ may sequester β-catenin, leading to the proteasomal degradation of this complex. As a result, a high activation of the WNT/β-catenin pathway could reduce PPARγ availability and vice versa. Second, WNT/β-catenin signaling induces the transcription of Cyclin D1, which promotes the recruitment of Histone deacetylase 3 (HDAC3) to PPARγ, inducing deacetylation, consequently, reducing the transcriptional activity of PPARγ. (B) Various naturally occurring compounds such as polyunsaturated fatty acids like eicosapentaenoic acid (EPA) and the ginsenoside Rb1 (GRb1), as well as synthetic compounds like Thiazolidines (TZD), can directly activate PPARγ. Upon activation, PPARγ interacts with retinoid-x-receptors (RXR) to induce the transcription of target genes. PPARγ may dimmish WNT/β-catenin signaling through the activation of glycogen synthase kinase-three-β (GSK3β)—an essential part of the β-catenin destruction complex. Moreover, PPARγ induces the transcription of WNT scavengers like secreted frizzled-related protein 2 and 5 (sFRP2/5), as well as secreted α-Klotho (α-sKlotho), which results in an inhibition of SOX9 and RUNX2 by scavenging WNT ligands such as WNT5a and WNT3a. The binding of dickkopf-1 (DKK1), another transcriptional target of PPARγ, to lipoprotein receptor-related protein 5 or 6 (LRP5/6) leads to a complex formation with the Kremen receptor (Kre), followed by its internalization and degradation, ultimately inhibiting Wnt/β-catenin signal transduction.