Figure 1. Stress‐related mechanisms leading to heart failure in congenital heart disease (CHD).
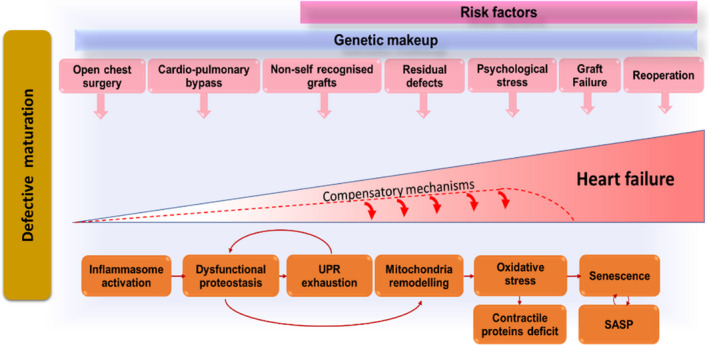
Congenitally defective cardiomyocytes are immature, fragile, and susceptible to stress‐induced damage. Cardiac cells (especially stromal cells, which have immunomodulatory roles) respond to the early and repeated stress by activating the inflammasome. Stress‐induced response pathways could attempt to reduce protein synthesis and increase the cellular capacity for protein folding and degradation. With unremitting stress, proteostasis becomes overloaded, resulting in misfolded protein accumulation in subcellular compartments, including the mitochondria, where proteotoxic stress can incite reactive oxygen species production. An increasing number of cells become senescent in the heart with CHD and transfer proteostatic stress to neighboring cells through secretion of inflammatory chemokines and misfolded peptides. This vicious cycle will lead to cardiomyocyte loss, microvascular rarefaction, and fibrocalcific interstitial remodeling of the heart, compromising myocardial perfusion and contractile function. The stress from the defect and surgical trauma is unavoidable, but drugs able to improve proteostasis and/or interfere with the inflammasome could halt cardiac deterioration in patients with CHD, thus succeeding where conventional therapy has failed. Improvement in graft biocompatibility (eg, cellularization before implantation) may reduce the inflammatory reaction to the prosthesis. SASP indicates senescence associated secretory phenotype; and UPR, unfolded protein response.
