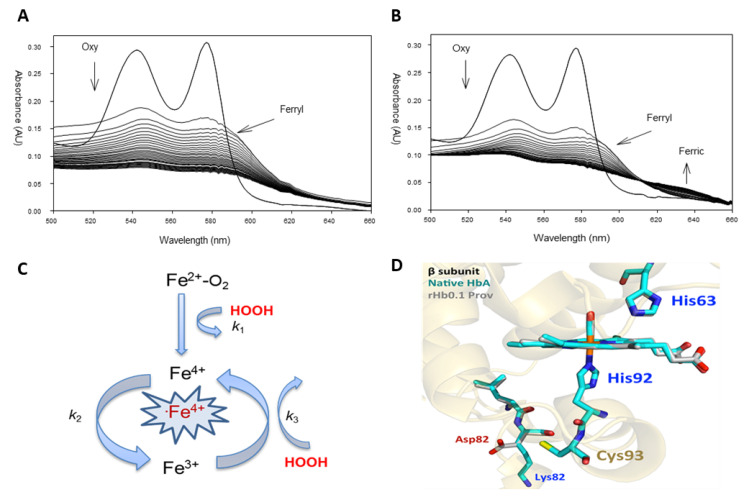
Figure 1.
Pseudoperoxidase activity of hemoglobin Providence. (A) Spectral changes in wild-type hemoglobin A (β82K) (arrows pointing downward indicate the direction of the reaction and the conversion of the oxy/ferrous (two main peaks; 541 and 577 nm) to ferryl heme (two main peaks; 545 and 580 nm)). (B) Hemoglobin Providence (βK82D) during oxidation by hydrogen peroxide. (C) A model represents the pseudoperoxidase activity of hemoglobin. (D) Comparison of the active sites, EF corner, F-helix, and C-terminus of β subunits in HbA (cyan, 2DN3) and rHb0.1/βK82D (rHb0.1 Prov) (gray, 5SW7). The drawing was made by MOL Molecular Graphics System, version 2.0 (Schrodinger, LLC) (New York, NY, USA). Panels A, B, and C were reprinted with permission from [14,17].