Abstract
Background
Total liquid ventilation (TLV) has been shown to prevent neurological damage though ultrafast cooling in animal models of cardiac arrest. We investigated whether its neuroprotective effect could be explained by mitigation of early inflammatory events.
Methods and Results
Rabbits were submitted to 10 minutes of ventricular fibrillation. After resuscitation, they underwent normothermic follow‐up (control) or ultrafast cooling by TLV and hypothermia maintenance for 3 hours (TLV). Immune response, survival, and neurological dysfunction were assessed for 3 days. TLV improved neurological recovery and reduced cerebral lesions and leukocyte infiltration as compared with control (eg, neurological dysfunction score=34±6 versus 66±6% at day 1, respectively). TLV also significantly reduced interleukin‐6 blood levels during the hypothermic episode (298±303 versus 991±471 pg/mL in TLV versus control at 3 hours after resuscitation, respectively), but not after rewarming (752±563 versus 741±219 pg/mL in TLV versus control at 6 hours after resuscitation, respectively). In vitro assays confirmed the high temperature sensitivity of interleukin‐6 secretion. Conversely, TLV did not modify circulating high‐mobility group box 1 levels or immune cell recruitment into the peripheral circulation. The link between interleukin‐6 early transcripts (<8 hours) and neurological outcome in a subpopulation of the previously described Epo‐ACR‐02 (High Dose of Erythropoietin Analogue After Cardiac Arrest) trial confirmed the importance of this cytokine at the early stages as compared with delayed stages (>8 hours).
Conclusions
The neuroprotective effect of hypothermic TLV was associated with a mitigation of humoral interleukin‐6 response. A temperature‐dependent attenuation of immune cell reactivity during the early phase of the post–cardiac arrest syndrome could explain the potent effect of rapid hypothermia.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00999583.
Keywords: critical care, liquid ventilation, therapeutic hypothermia
Subject Categories: Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- BBB
blood‐brain barrier
- CA
cardiac arrest
- HMGB1
high mobility group box 1
- PS100B
protein S100B
- ROSC
resumption of spontaneous circulation
- TLV
total liquid ventilation
Clinical Perspective
What Is New?
We have already demonstrated that total liquid ventilation could be efficient to prevent neurological damages though ultrafast cooling properties in animal models of cardiac arrest; in this study, we demonstrated that total liquid ventilation–induced hypothermia also transiently modulates the acute inflammatory response, which likely contributes to its neuroprotective effect.
Surprisingly, this anti‐inflammatory effect was not caused by a limitation of immediate cell death after resuscitation but by a direct downregulation of the subsequent innate immune response and interleukin‐6 early release.
This further supports the importance of early interventions for cooling and inflammation mitigation in clinical practice after cardiac arrest.
What Are the Clinical Implications?
Our results show that the inflammatory events occurring during the first 3 hours after resuscitation play a major role in the pathophysiology of the post–cardiac arrest syndrome.
From a mechanistic point of view, it further emphasizes the role played by the early critical period of immune overstimulation after cardiac arrest.
Hypothermia might provide a transient tolerance to these proinflammatory stimuli and prevent later dysregulation of the immune response; this is consistent with several experimental and clinical reports demonstrating an optimal window of efficiency of rapid systemic hypothermia during the first 2 to 4 hours after resuscitation.
Ischemia‐reperfusion is responsible for neurological disabilities after cardiac arrest (CA) and resuscitation. 1 To improve the patient’s outcome, targeted temperature management is recommended, even if the ideal target temperature is still debated. 2 In laboratory studies, strategies aiming at fastening cooling induction provide promising results to further improve the ultimate neurological outcome. 3 , 4 Some clinical data are also supporting the importance of early achievement of target temperature in humans after resuscitation. 5 Accordingly, our team developed an original technique to reduce the time to target temperature through the total liquid ventilation (TLV) of the lungs with temperature‐controlled perfluorocarbons. We previously demonstrated its safety and efficiency for whole body cooling and prevention of the post–cardiac arrest syndrome in animal models. 6 , 7
In experimental studies, hypothermia was shown to mitigate the inflammatory response induced by ischemia‐reperfusion through inhibition of proinflammatory cytokine generation and attenuation of neuroinflammation. 8 , 9 However, the anti‐inflammatory benefit of hypothermia is still controversial in clinical settings. 10 This discrepancy might be explained by the delayed institution of hypothermia in clinical conditions, with target temperature often achieved several hours after resuscitation. Thus, patients might not benefit from the effect of rapid hypothermia on the triggering phase of inflammation, which remains poorly explored. Our hypothesis is that hypothermia mitigates the early inflammatory response when achieved rapidly, which could contribute to the greater effect of rapid cooling after CA. 6 , 7 In addition, several studies support the fact that inflammation is a major contributor of the propagation of neuronal death after ischemia‐reperfusion. 11 , 12 For instance, recent clinical studies demonstrated the importance of the systemic inflammatory response on the patient’s outcome, with a close correlation between interleukin‐6 circulating levels and mortality. 11 , 12 On the bench side, interleukin‐6 signaling was further shown to contribute to no reflow, 13 blood‐brain barrier (BBB) dysfunction after brain ischemia, 14 ultimately exacerbating neuroinflammation. In addition, previous reports from our group showed that the neuroprotective effects of TLV are associated with preservation of BBB integrity and normalization of cerebral blood flow in the acute phase following resuscitation. 8 , 15 Those effects might represent direct consequences of the anti‐inflammatory potential of the procedure.
Accordingly, we sought to evaluate the effect of ultrafast hypothermia with TLV on the inflammatory process of the post‐CA syndrome, in line with the ultimate neuroprotective properties. We also investigated a putative link with early cell death reduction through the assessment of the proinflammatory mediator high‐mobility group box 1 (HMGB1) as a key damage‐associated molecular pattern after CA. 16 , 17 Finally, to support our experimental findings, we further evaluated the link between humoral response and neurological outcome in clinical conditions in patients included in the previously described Epo‐ACR‐02 (High Dose of Erythropoietin Analogue After Cardiac Arrest) trial population.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The animal instrumentation and ensuing experiments were conducted in accordance with official French regulations after approval by the local ethics committee (ComEth AnSES/ENVA/UPEC no. 16; project 2017111414547261).
Animal Preparation and Clinical Follow‐Up
Male New Zealand rabbits (2.5–3.0 kg) were anesthetized using zolazepam, tiletamine, and pentobarbital (all 20–30 mg/kg IV), as well as buprenorphine (30 μg/kg IV). We did not include females since they could present different hormonal status. They were intubated and mechanically ventilated using a volume‐controlled ventilator (Alpha Lab‐Minerve, Esternay, France), with a tidal volume set at 12 mL/kg and FiO2 at 0.21, respectively. Respiratory rate was initially set at 28 cycles/min and then adjusted to maintain end‐tidal CO2 around 35 to 40 mm Hg. After administration of rocuronium bromide (1 mg/kg IV), 2 electrodes were implanted on the inner muscular wall and inserted into the esophagus for subsequent induction of ventricular fibrillation. Body temperatures and ECG were followed throughout the experiments, as well as systemic blood pressure through a catheter inserted into the ear artery. After a period of stabilization, an alternative current (12 V, 4 mA; 2.5 minutes) was delivered between the electrodes to induce ventricular fibrillation. Concomitantly, mechanical ventilation was stopped. After 10 minutes of untreated fibrillation, cardiopulmonary resuscitation was performed using external manual cardiac massage (200 external chest compressions/min), electric defibrillation (10 J/kg), and intravenous administration of epinephrine (15 μg/kg IV). During cardiopulmonary resuscitation, animals were reconnected to the ventilator that was switched to a respiratory mode allowing to deliver a continuous flow of oxygen (FiO2=100%). Animals were excluded if resumption of spontaneous circulation (ROSC) was not achieved in <10 minutes after the beginning of cardiopulmonary resuscitation. After ROSC, epinephrine administration was allowed to reach a target mean blood pressure of 70 mm Hg. After 6 hours of follow‐up, animals were awakened for subsequent neurological and survival follow‐up for 3 days. Neurological dysfunction was evaluated blindly and daily using a clinical score previously validated in rabbits (0%=normal, 100%=death). 6 , 8 For ethical considerations, animals eliciting a neurological dysfunction score >80% at 24 hours or 60% at 48 hours were prematurely euthanized. All surviving animals were euthanized at the end of the 3‐day follow‐up for histological analysis.
Experimental Protocol
As illustrated in Figure 1, animals were divided into 3 experimental groups. The first group was submitted to animal preparation and subsequent follow‐up with no CA (sham group, n=5). The 2 other groups underwent electrical induction of CA and then were randomly allocated to a control (n=6) or TLV (n=6) procedure after ROSC. The control group did not receive any additional procedure and was maintained under normothermic conditions with thermal pads until awakening. In the TLV group, ultrafast cooling was induced by TLV started 15 minutes after ROSC. The lungs were filled with 10 mL/kg of perflubron (Exfluor, Round Rock, TX), with an initial temperature of 20°C and a progressive increase to 33°C. The liquid ventilator was set to a tidal volume of 10 mL/kg, a respiratory frequency of 8 cycles/min, and a positive end‐expiratory pressure of 2 cm H2O. We used a previously described algorithm with a volume‐ and pressure‐controlled liquid ventilation mode. After 20 minutes of TLV and achievement of the hypothermic target temperature of 33°C, animals were weaned by prolonged exhalation at −15 cm H2O. The liquid ventilator was then disconnected and animals were shifted to conventional mechanical ventilation. Hypothermia was further maintained by cold blankets for 3 hours. Animals were then slowly rewarmed with infrared lights and thermal pads for 4 hours before weaning from conventional ventilation and awakening. At the end of procedure, animals were awakened for survival follow‐up. In both groups, they were housed in a closed cage enriched in oxygen for 24 hours and received analgesics (buprenorphine, 30 μg/kg IM) every day for 3 days.
Figure 1. Experimental protocol, temperature, and hemodynamic parameters.
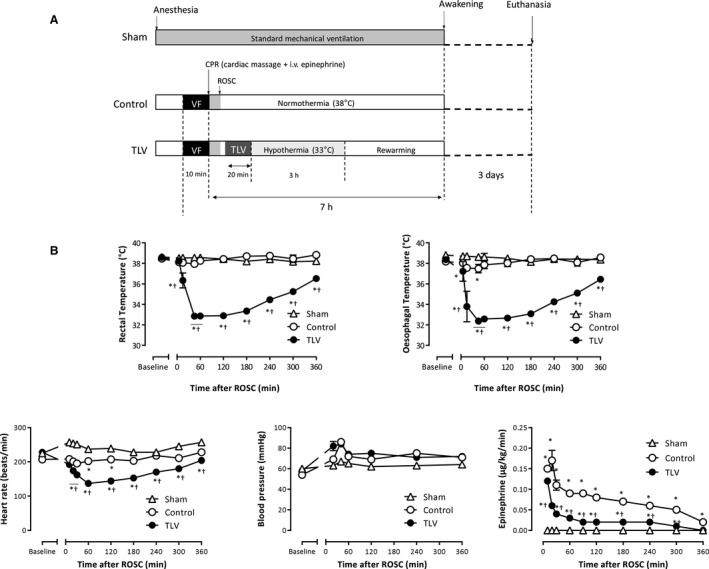
A, Experimental protocol illustrating the different procedures. After resuscitation, animals were randomly assigned to a conventional follow‐up under normothermic mechanical ventilation (control group, n=6) or to hypothermic total liquid ventilation (TLV; n=6). A third group was submitted to a sham procedure with no cardiac arrest (n=5). Animals were excluded if resumption of spontaneous circulation (ROSC) was not achieved in <10 minutes after the beginning of cardiopulmonary resuscitation. B, Esophageal and rectal temperatures, mean arterial pressure, heart rate, and epinephrine infusion rate throughout the experimental protocol in the different groups. Data are expressed as mean±SEM. Statistical comparisons were only made between groups but not among different time points; *P<0.05 vs corresponding sham; † P<0.05 vs corresponding Control. VF indicates ventricular fibrillation.
Evaluation of Blood Inflammatory Markers and Leukocytes
Using ELISA, blood levels of HMGB1 (Abbexa, Milton, United Kingdom) and protein S100B (PS100B; FineTest, Wuhan, China) were evaluated as markers of general and cerebral cell death. Interleukin‐6 (R&D Systems, Minneapolis, MN), interleukin‐1β (R&D Systems), tumor necrosis factor‐α (TNF‐α, R&D Systems) and interleukin‐10 (Elabscience, Houston, TX) were also measured using the same method. In the control and TLV groups, blood samples were also prepared for flow cytometry and leukocyte counts, as described in additional material (Data S1).
Histological Analyses
At the end of the 3‐day follow‐up, rabbits were euthanized and perfused with 5% paraformaldehyde through both carotids. Brains were then removed, postfixed with paraformaldehyde, and embedded in paraffin. Brain coronal sections were stained with hematoxylin and eosin. Neurons were labeled by Fluorojade C staining (Merck Millipore, Burlington, MA). For each animal and each analyzed region, positive neurons were blindly counted in 5 nonoverlapping fields of the same section at ×200 magnification. Data were expressed as the average value of the 5 counts.
Immunohistochemical analyses were also performed to quantify the invasion of the brain by peripheral immune cells. Neutrophils and T cells were detected with a monoclonal antibody against a cell surface antigen that is expressed by a subset of T cells, thymocytes, and neutrophils (diluted 1:100; RPN3/57, BioRad, Hercules, CA).
Whole Blood Stimulation Experiment
To evaluate the proper effect of hypothermia on immune cell reactivity, leukocytes were stimulated in vitro with toll‐like receptor ligands and incubated at different temperatures. Accordingly, arterial blood was withdrawn from healthy rabbits using heparinized syringes and diluted 1:1 with Roswell Park Memorial Institute medium. Then samples were immediately stimulated with lipopolysaccharide (100 ng/mL; Sigma‐Aldrich, St. Louis, MO) and incubated either at 33°C or at 38°C for 2 or 6 hours. At the end of the incubation period, samples were centrifuged at 600g for 10 minutes at 4°C. Supernatant was stored at −80°C, and levels of interleukin‐6, interleukin‐1β, and TNF‐α were determined by ELISA, according to the manufacturer’s instructions (R&D Systems).
Evaluation of Cytokine Expression in Human Samples After CA
To further evaluate clinical relevance of our results, we leveraged available data from a published clinical trial initially designed to evaluate the effect of erythropoietin after out‐of‐hospital CA (ancillary study of NCT00999583). In the corresponding study, blood samples were collected in 69 comatose survivors of CA at hospital admission, and 1 and 3 days after resuscitation (the exact time of sampling was registered) and exhaustive analysis of blood gene expression (>34 000 genes; HumanHT‐12 V4 BeadChip, Illumina, San Diego, CA;) was performed. 18 Patients were classified into 2 categories representing neurological favorable outcome (Cerebral Performance Category=1–2) versus unfavorable outcome (Cerebral Performance Category >2) at day 60 after CA. We extracted data corresponding to the cytokine transcription levels, time of sampling after CA, and neurological outcome to compare interleukin‐6, TNF‐α, and interleukin‐1β expression between patients with good versus bad neurological outcome. The samples were grouped for comparison on the basis of the time they were collected after CA (ie, within 0–8 hours after CA, n=25 versus 33 in the group with good versus bad outcome, respectively, and within 8–36 hours after CA, n=28 versus 35 in the group with good versus bad outcome, respectively).
Statistical Analysis
Data were expressed as mean±SE unless otherwise stated. Continuous variables were compared between groups using a 2‐way ANOVA for repeated measures, considering group, time, and interaction terms. If necessary, post hoc analyses were performed at each time point using a Student t test with Bonferroni correction. Neurological dysfunction, histological scores, and cytokine transcription level in patients were compared between groups using a nonparametric Mann–Whitney test. For the cytokine transcription level in patients, 2 different comparisons were made, that is, for samples withdrawn <8 hours or 8 to 36 hours after resuscitation. Correlation studies among clinical score and cytokine blood levels were performed using nonparametric Spearman analysis. The primary outcome was the neurological dysfunction score, as a marker of TLV‐induced neuroprotection. We had to include 5 animals in each group to evidence a 40% reduction in neurological dysfunction at day 1 (β=0.1). In the control and TLV groups, 6 animals were included to account for possible false‐positive results when testing numerous secondary outcomes. Statistical analyses were performed using Prism 6 (GraphPad Software, La Jolla, CA). Significant differences were determined at P value ≤0.05.
Results
TLV Provided Rapid Mild Hypothermia Without Hemodynamic Adverse Effect
Seventeen rabbits successfully underwent the whole protocol of the main study (n=6, 6, and 5 in the control, TLV and sham groups, respectively). Five animals were excluded immediately after CA because of a lack of successful resuscitation before group allocation. In control and TLV groups, other animals were successfully resuscitated with similar times to ROSC after CA (3.9±1.2 and 2.9±0.7 minutes in the control and TLV groups, respectively). In the TLV group, the body target temperature of 33°C was achieved within 30 minutes after resuscitation (ie, 15 minutes after the institution of TLV), whereas it was maintained at 38°C during the whole procedure in the control group (Figure 1B). During the hypothermic period, mean arterial pressure did not differ, but heart rate was decreased in the TLV group versus control. The amount of epinephrine required to prevent hypotension was significantly lower in the TLV group as compared with control. No significant difference in conventional biochemical parameters between the 2 groups was evidenced before and after CA (Table S1).
TLV Mitigates the Immune Response After CA Through a Specific Reduction in IL‐6 Blood Levels During the Hypothermic Episode Itself
As illustrated in Figure 2A, blood levels of HMGB1 increased rapidly after resuscitation in both the control and TLV groups, but there was no difference between the 2 groups at 30 minutes after ROSC (30±7, 35±13, and 10±0.1 ng/mL in the control, TLV, and sham groups, respectively), suggesting a similar magnitude for immediate cell death after resuscitation. Likewise, TLV did not modify the rise in PS100B plasma levels as compared with the control group during the first 3 hours after ROSC. Indeed, baseline concentrations of PS100B increased from 124±62% and 92±37% at 180 minutes after ROSC in the TLV and control groups, as compared with baseline values (1165±310 and 1080±181 pg/mL, respectively).
Figure 2. Early release of tissular damage and inflammatory markers in the different groups.
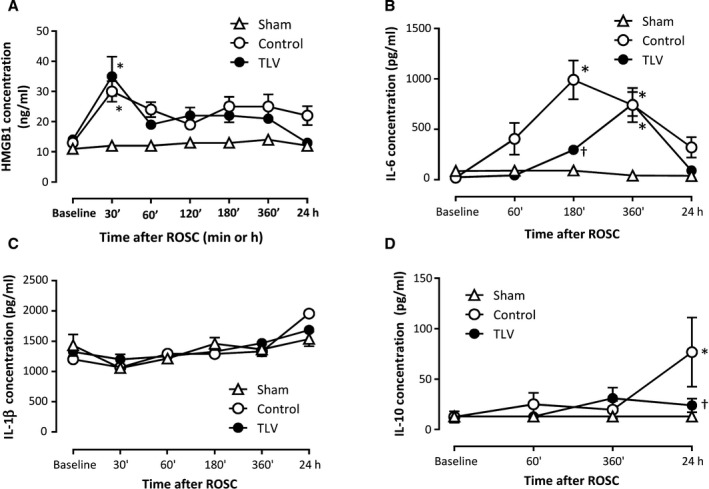
Blood concentrations of high‐mobility group box 1 (HMGB1; A), interleukin‐6 (B), interleukin‐1β (C), and interleukin‐10 (D) throughout the procedure, (n=5, 6 and 6 in sham, control, and TLV groups, respectively). Data are expressed as mean±SEM. Statistical comparisons were only made between groups but not among different time points; *P<0.05 vs corresponding sham; † P<0.05 vs corresponding control. ROSC indicates resumption of spontaneous circulation; and TLV, total liquid ventilation.
As illustrated in Figure 2B, a striking increase in plasma interleukin‐6 level was observed after CA in the control versus the sham group. Conversely, no difference was observed regarding TNF‐α release (concentrations below the limit of quantification throughout the follow‐up) or interleukin‐1β blood levels. In the TLV group, interleukin‐6 blood levels were significantly decreased during the hypothermic episode as compared with control conditions (180 minutes after CA). Conversely, interleukin‐6 levels were no more reduced as compared with control after rewarming (Figure 2B).
In line with this modification of the early proinflammatory response, TLV reduced the blood levels of interleukin‐10 anti‐inflammatory cytokine at 24 hours after CA as compared with control. This suggests that TLV not only delays the onset of interleukin‐6 signaling until rewarming but also mitigates the compensatory anti‐inflammatory response after CA.
Despite the above‐mentioned effects, leukocytes similarly increased in the TLV versus the control group after CA (Figure S1). The leukocytosis was mainly caused by a mobilization of granulocytes, whereas all lymphocytes population subsets (including T‐helper cells, cytotoxic cells, and B lymphocytes) showed a slight decrease over time. Hypothermia modified neither the influx of granulocytes to the peripheral circulation nor the granulocyte/lymphocyte proportions after CA.
Ultrafast Hypothermia Improved the Ultimate Neurological Outcome
Ultrafast cooling by TLV was associated with a dramatic improvement of the neurological recovery as compared with control conditions. As shown in Figure 3A, the neurological dysfunction score achieved 34±6% at day 1 in the TLV group as compared with 66±6% in the control group. In the latter group, all animals still elicited severe neurological dysfunction at day 2, leading to premature euthanasia as prospectively decided with the ethical committee (neurological dysfunction score >60%). In the TLV group, all animals had a lower neurological dysfunction score at day 2 (21±5%) versus day 1, allowing further follow‐up until day 3. The resulting impact on survival outcome is illustrated by Figure 3B. To confirm the neuroprotective effect of TLV, histopathological evaluations were performed using Fluorojade C staining for the identification of degenerating neurons. A significant reduction of neuronal injuries was confirmed in parasagittal cortex and hippocampus in the TLV group as compared with control (eg, 4.5±4.8 versus 21.3±15.4 degenerating cells/field in the hippocampus, respectively) (Figure 3C and 3D).
Figure 3. Neurological dysfunction score and histopathological morphology.
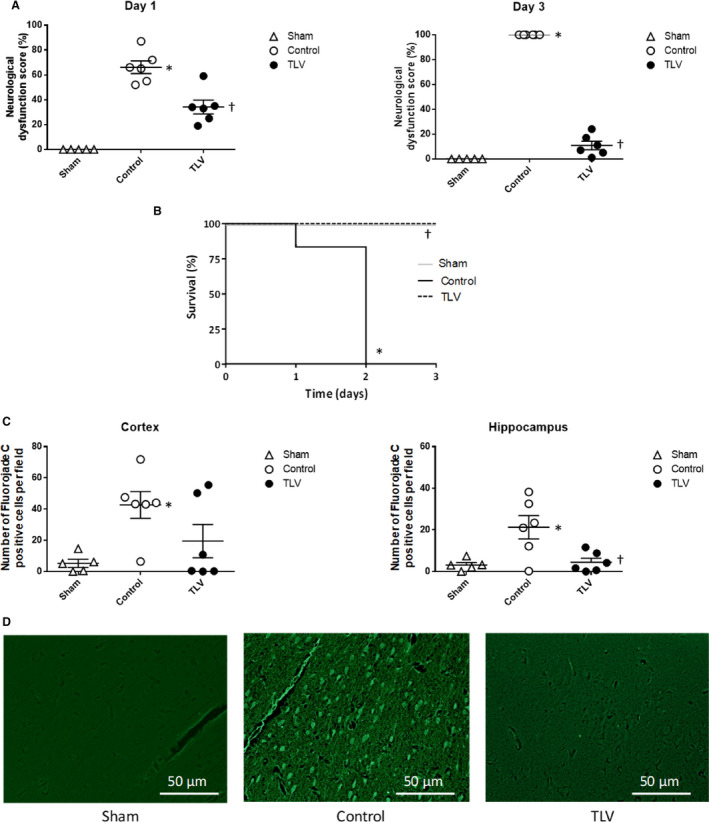
A, Neurological dysfunction at days 1 and 3 following resuscitation in the different experimental groups (0%=lack of dysfunction; 100%=death). Circles represent individual values. Lines represent mean values and corresponding standard error of the mean. B, Kaplan–Meyer survival curves. All premature death in the control group are related to anticipated euthanasia for ethical considerations. C, Number of degenerating neurons per field, as defined by positive Fluorojade C cells. Numbers are expressed as mean number per analyzed field, in parasagittal cortex and hippocampus. Numbers are mean values from 5 field per rabbit and area. D, Typical histological appearance of the cortex after Fluorojade C staining, showing no or few degenerating neurons in sham and TLV groups, as compared with frequent degenerating neurons in the control group. Data are expressed as mean±SEM. *P<0.05 vs corresponding sham; † P<0.05 vs corresponding control. TLV indicates total liquid ventilation; n=5, 6, and 6 in sham, control, and TLV groups, respectively.
As shown in Figure 4, immunohistochemical analyses revealed an increase in the number of leukocytes into the brain parenchyma of control animals as compared with sham animals. This infiltration was significantly reduced in the TLV group.
Figure 4. Brain invasion by peripheral immune cells.
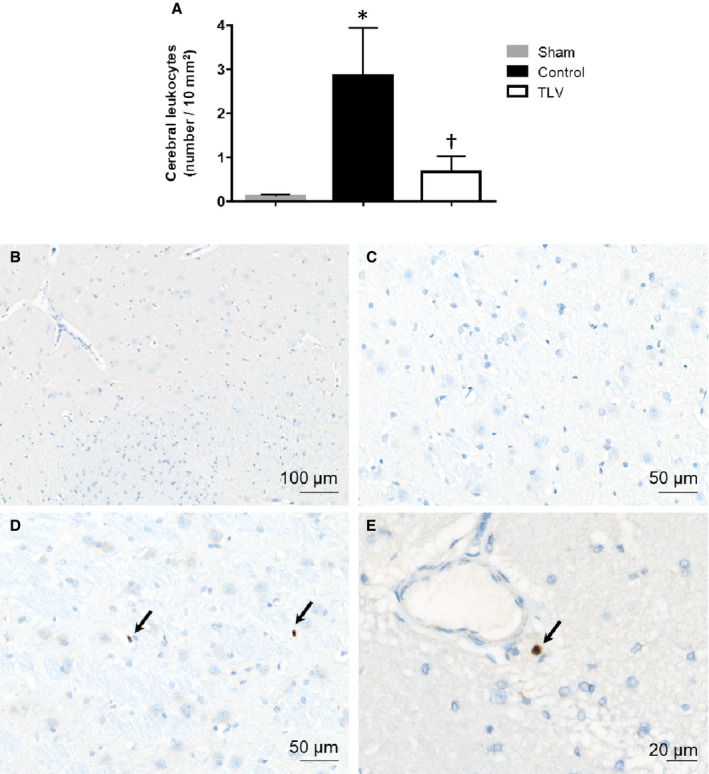
A, Number of leukocytes (lymphocytes and neutrophils) localized into the cerebral parenchyma in each group. B through D, Morphological appearance of the immunohistochemical staining used for leukocyte identification. No positive cells were observed in sham animals (B and C). In the control and TLV groups, leukocytes (black arrows) were observed into the brain parenchyma close to blood vessels (D and E in a rabbit from the control group). Data are expressed as mean±SEM. *P<0.05 vs corresponding sham; † P<0.05 vs corresponding control.
Hypothermia Inhibits the Immune Cells Reactivity In Vitro
To assess the proper effect of mild hypothermia on peripheral blood cell secretory activity, additional in vitro experiments were completed for the evaluation of immune cell reactivity. For this purpose, proinflammatory cytokine production was determined at either 33°C or 38°C after stimulation with lipopolysaccharide (Figure 5). Interleukin‐6, interleukin‐1β, and TNF‐α concentrations increased as early as 2 hours after stimulation in comparison with baseline, but the increase was reduced at 33°C versus 38°C. Interleukin‐6 production was divided by 3 compared with that at 38°C, independently from the duration of incubation.
Figure 5. Cytokine production by peripheral blood cells stimulated with lipolysaccharide (lipopolysaccharide) at 33°C vs 38°C.
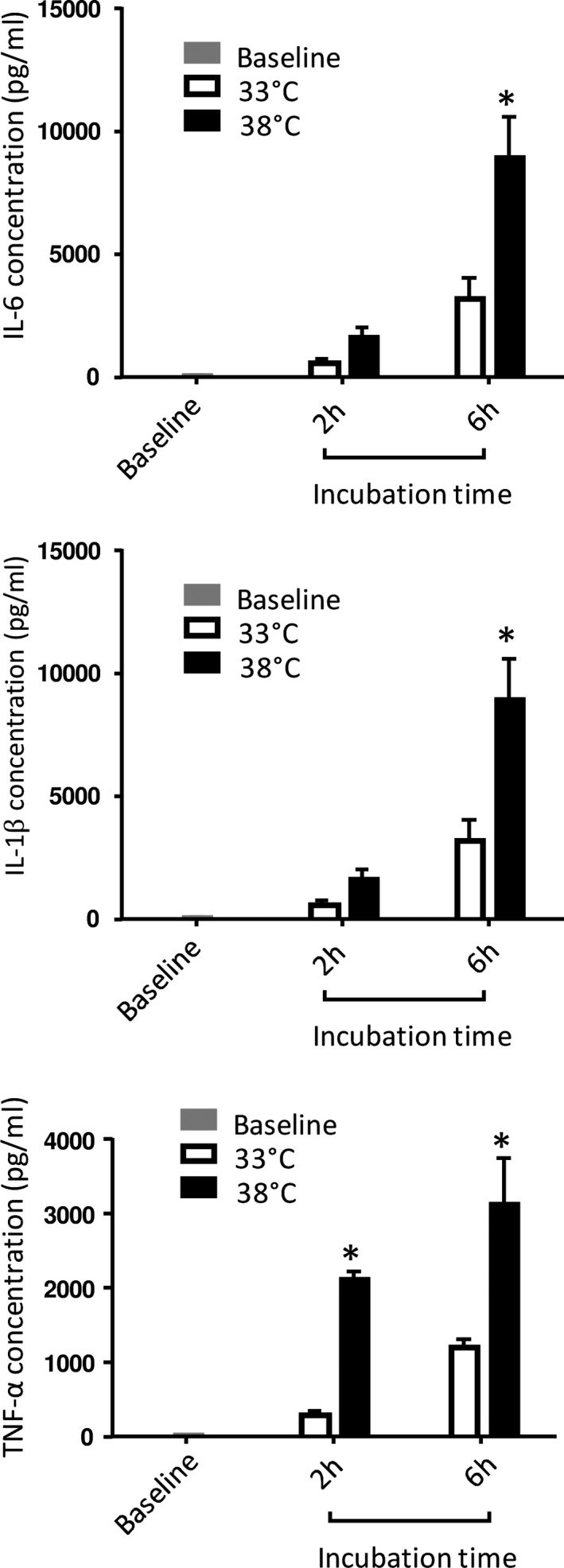
Fresh whole blood from rabbits was incubated during 2 to 6 hours after lipopolysaccharide adjunction. Experiments were done at either 33°C and 38°C (n=6 for each condition). Interleukin‐6 data are expressed as mean±SEM. Interleukin‐1β and tumor necrosis factor (TNF)‐α concentrations were then measured in the plasma. *P<0.05 vs corresponding value at 33°C.
Early Immune Response and Interleukin‐6 Transcripts Are Also Associated With Ultimate Outcome in Humans
To confirm the importance of early versus late interleukin‐6 levels after CA, we analyzed mRNA blood levels of interleukin‐6, interleukin‐1β, and TNF‐α in patients. 18 We compared the values of these cytokines when evaluated from samples withdrawn early (<8 hours) as compared with later ones (8–30 hours) after CA in patients with poor versus favorable outcome (Cerebral Performance Category 1–2 versus Cerebral Performance Category >2). As shown in Figure S2, interleukin‐6 levels were significantly different in patients with good versus bad ultimate outcome at early time points (ie, <8 hours after CA) but not later after ROSC (ie, 8–36 hours after CA). Interleukin‐1β and TNF‐α were similar among patients with poor versus bad outcome, regardless of the timing of investigation. This again highlights the functional importance of interleukin‐6 during the early phase after CA.
Discussion
In this study, we investigated the effect of early hypothermia induced by TLV on the initiation of the inflammatory response following CA. Surprisingly, the anti‐inflammatory properties of TLV were not mediated by reduction of early cell death and subsequent release of danger signals. Early hypothermia rather uncoupled the acute immune signalization and the ultimate deleterious effects after CA. As there was no difference regarding mobilization of immune cells toward peripheral circulation among groups, we assumed that hypothermia simply acted through direct downregulation of immune cell reactivity, which was further corroborated by in vitro results of whole blood stimulation assays.
As stated above, hypothermia instituted rapidly after ROSC did not reduce early cell death after CA as compared with normothermic animals, as evidenced by similar blood levels of HMGB1 and PS100B. Indeed, HMGB1 is known to be a prototypical damage‐associated molecular pattern released by a great variety of cells, whereas PS100B mainly reflects cerebral cell death. Since hypothermia was well shown to prevent excitotoxicity 19 or to dampen reactive oxygen species generation, 20 , 21 this suggests that cells have already endured irreversible lesions during the no‐flow period, and early cell death after CA might not be prevented by postreperfusion interventions. Conversely, hypothermia seemed to partially block the inflammatory consequences of cell necrosis. A lower systemic humoral immune response was indeed evidenced regarding interleukin‐6 blood levels, in line with improved neurological outcome at the end of the follow‐up. It was associated with a reduction of delayed neuronal lesions as shown by Fluorojade C analyses. Thus, in our experimental setting, hypothermia appeared to uncouple the early processes triggered by ischemia‐reperfusion from their delayed consequences, that is, secondary cerebral lesions.
Unexpectedly, this uncoupling did not require a total blunt of inflammatory events as interleukin‐6 blood levels returned to control value in the TLV group during the rewarming period. Hypothermia delayed the secretion of proinflammatory mediators rather than completely suppressing it. This is fully in line with the results of the in vitro experiments, showing a direct relationship between temperature and interleukin‐6 secretion by blood immune cells. Indeed, a major reduction in cytokine concentration was observed in blood samples incubated at 33°C versus 38°C. The transient repression exerted by hypothermia on the systemic inflammation in the present rabbit study then suggests that inflammatory events occurring during the first 3 hours after ROSC play a major role in the pathophysiology of the post‐CA syndrome. That is why we attempted to determine whether cytokine blood levels are also linked with the ultimate patient outcome at rather early versus delayed time points in clinical conditions. We had the opportunity to evaluate this hypothesis in the previously reported Epo‐ACR‐02 population, in which we tested the transcriptomic signature after cardiac arrest. 18 The results further corroborated our hypothesis, showing a correlation between interleukin‐6 transcript levels and neurological recovery only at very early but no later time points after ROSC in patients (ie, 0–8 versus 8–36 hours). From a mechanistic point of view, it further emphasizes the importance of an early critical period of immune overstimulation after cardiac arrest. Hypothermia might provide a transient tolerance to these proinflammatory stimuli and prevent later dysregulation of the immune response. This is also consistent with several experimental and clinical reports demonstrating an optimal window of efficiency of rapid hypothermia during the first 2 to 4 hours after ROSC. 3 , 4 , 22 , 23 For instance, a recent substudy of the Resuscitation Outcomes Consortium Continuous Chest Compressions trial showed higher benefits of hypothermia after CA when initiated during the first 122 minutes after ROSC. 23
Importantly, early hypothermia also attenuated the delayed compensatory anti‐inflammatory response occurring after rewarming in our experimental conditions, as suggested by the decrease in interleukin‐10 concentrations 24 hours after resuscitation in the TLV group. This secondary anti‐inflammatory reaction is well described in patients, who tend to spontaneously develop an immunodeficiency state called endotoxin tolerance, characterized by impaired leukocyte reactivity to infectious agents and thus enhanced sensitivity to nosocomial infections. 24 , 25 By diminishing the vigor of the initial proinflammatory reaction, early hypothermia might help to maintain the immunological balance after rewarming.
As stated above, another important finding is the predominant interleukin‐6 response after CA in the present study, while we did not observe a significant increase in interleukin‐1β or TNF‐α blood levels or transcripts. Though we do not have the direct demonstration of the causal role in the protective effect of TLV, several hypotheses can be raised regarding previous findings from our groups and others. 10 , 21 , 22 , 23 For instance, interleukin‐6 has been shown to increase BBB permeability by modulating the tight junction proteins 14 and to promote peripheral cell infiltration into tissues during acute inflammation. 26 Thus, downregulation of interleukin‐6 secretion could contribute to BBB preservation, a known benefit of TLV, previously highlighted by our team, 8 during a period of exacerbated susceptibility. Interleukin‐6 has also been shown to stimulate coagulation and microvascular thrombosis, and thus could be responsible for no‐reflow phenomenon. Figure 6 illustrates a schematic representation of this putative action mechanism of TLV on acute inflammation, summarizing the putative link between cerebral microvascular disorders and interleukin‐6 signaling. 8 , 13 , 14 , 15 , 27 , 28 , 29
Figure 6. Schematic representation of a putative mechanisms of protection of early hypothermia induced by total liquid ventilation (TLV) after cardiac arrest, with a focus on the early inflammation reaction.
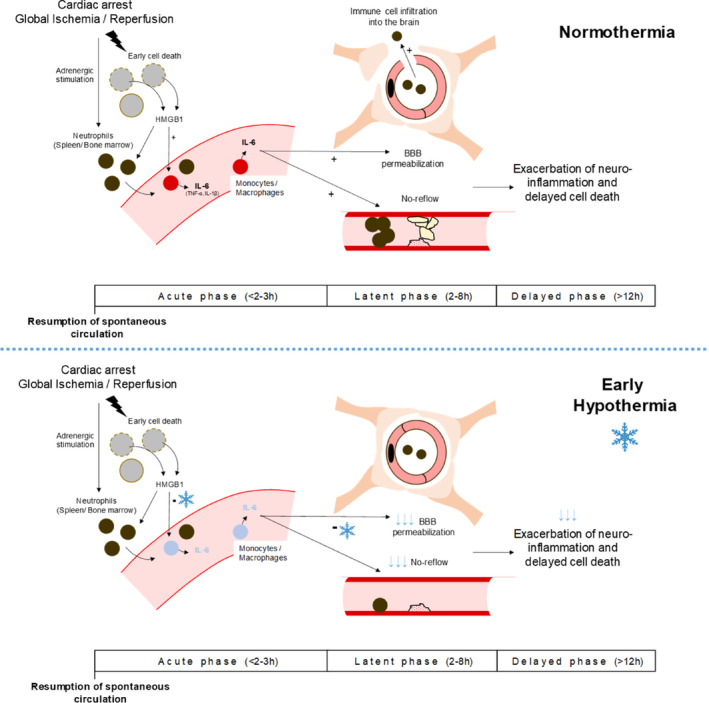
Beyond the results of the present study, the summarized assumptions are also based on previous findings of our group and others. First, it was previously demonstrated that TLV reduces the early permeabilization of the blood‐brain barrier (BBB) 8 and prevents the cerebral hemodynamic disturbance. 15 Second, the link between interleukin‐6, BBB dysfunction and no‐reflow development has also been previously demonstrated in animal models of brain ischemia. 13 , 14 Third, it is well shown that interleukin‐6 secretion by immune cells is an immediate response to danger signals such as high mobility group box 1 (HMGB1) that are released after ischemic injury. 27 , 28 , 29 That is why a global mechanistic hypothesis could be proposed with a putative direct effect of early hypothermia on interleukin‐6 secretion during the acute phase after cardiac arrest, leading to a preservation of BBB dysfunction, no‐reflow extension, and neurological lesions exacerbation.
Finally, this study also provides detailed information regarding the time course of the acute inflammatory events contributing to post‐CA syndrome. In our experimental setting, a temporal pattern of plasma interleukin‐6 increase was in favor of a strong but relatively short proinflammatory humoral response. Maximal interleukin‐6 concentrations were reached 3 hours after ROSC, with levels similar to those reported in sepsis, 30 but there was no longer a significant difference between the control and TLV groups at day 1 of follow‐up. This kinetic might explain the failure of clinical studies to demonstrate the anti‐inflammatory properties of mild hypothermia as they generally focused on later time points after resuscitation. Furthermore, during the first 24 hours after resuscitation, the proinflammatory signalization seemed specifically mediated by elevation of interleukin‐6 levels, with very little contribution of other proinflammatory cytokines such as TNF‐α and IL‐1β. Although our observations were conducted at a systemic level and deserve additional evaluation in brain tissue, it strongly suggests independent regulation of these proinflammatory cytokines in this context. It was unexpected as both TNF‐α and interleukin‐1β were reported to be involved early in the post‐CA inflammatory cascade, primarily released by leukocytes and endothelial cells. 24 , 25 , 31 This discrepancy, as well as late elevation in interleukin‐6 plasma concentration after CA in patients, may reflect bacterial translocation and secondary development of a sepsis rather than direct inflammatory consequences of CA and should be addressed differently than by anti‐inflammatory interventions. In that regard, Adrie et al 24 reported frequent endotoxemia in patients following CA (endotoxin was detected in the plasma of 46% of patients on the first 2 days after CA).
Importantly, our study presents some limitations. First, hypothermia was maintained only for a 3‐hour period after CA, as compared with 24 to 36 hours, which is usually applied in the clinical arena. Our rationale was that early achievement of whole‐body hypothermia, only for a short period, is sufficient to provide potent neuroprotection, as well demonstrated in previous animal studies with TLV. 6 , 7 , 8 , 32 We chose to evaluate the mechanism underlying this particular modality of protection offered by hypothermia, which seems more “specific” to the action of very early cooling. Longer durations of hypothermia may be associated with more profound alteration of the immune response, such as immunoparalysis. Second, the rabbit immune response following acute ischemic insult may not exactly reflect the one that takes place in humans, such as already suspected in rodent models of inflammatory diseases. 30 Finally, the small sample sizes may lead to an insufficient statistical power to evidence small differences in some of the studied markers, such as HMGB1, PS100B, or interleukin‐1β.
In conclusion, we demonstrated that hypothermia, when achieved with TLV early after ROSC, transiently modulates the acute inflammatory response, which likely contributes to its neuroprotective effect. Surprisingly, this anti‐inflammatory effect did not rely on limitation of immediate ischemia‐reperfusion–mediated cell death but on direct downregulation of the subsequent innate immune response and interleukin‐6 early release. These findings support the importance of early interventions in clinical practice and rapid institution of hypothermia after CA.
Sources of Funding
This study was supported by the Agence Nationale pour la Recherche (COOLIVENT Grant).
Disclosures
Dr Tissier is named as inventor on a patent on cooling with liquid ventilation (US20120226337 A1). Dr Micheau declares owning patents on liquid ventilation (US Patents # 7,726,311; Preliminary US patent 61/838,896). Drs Kohlhauer, Micheau, and Tissier are shareholders of a start‐up company dedicated to the clinical research on total liquid ventilation (Orixha). The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
Figures S1–S2
Acknowledgments
The authors are greatly indebted to Dr Delphine Le Roux, Jean‐Luc Servely, and the BioPole Alfort for a high level of expertise and facilities for biological analyses.
(J Am Heart Assoc 2020;9:e017413 DOI: 10.1161/JAHA.120.017413.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017413
For Sources of Funding and Disclosures, see page 12.
References
- 1. Neumar RW, Nolan JE, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, et al. Post–cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Circulation. 2008;118:2452–2483. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. [DOI] [PubMed] [Google Scholar]
- 3. Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39:1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. [DOI] [PubMed] [Google Scholar]
- 5. Uribarri A, Bueno H, Pérez‐Castellanos A, Loughlin G, Sousa I, Viana‐Tejedor A, Fernández‐Avilés F. Impact of time to cooling initiation and time to target temperature in patients treated with hypothermia after cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2015;4:365–372. [DOI] [PubMed] [Google Scholar]
- 6. Chenoune M, Lidouren F, Adam C, Pons S, Darbera L, Bruneval P, Ghaleh B, Zini R, Dubois‐Randé J‐L, Carli P, et al. Ultrafast and whole‐body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits. Circulation. 2011;124:901–911, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohlhauer M, Boissady E, Lidouren F, de Rochefort L, Nadeau M, Rambaud J, Hutin A, Dubuisson R‐M, Guillot G, Pey P, et al. A new paradigm for lung‐conservative total liquid ventilation. EBioMedicine. 2019;52:102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohlhauer M, Lidouren F, Remy‐Jouet I, Mongardon N, Adam C, Bruneval P, Hocini H, Levy Y, Blengio F, Carli P, et al. Hypothermic total liquid ventilation is highly protective through cerebral hemodynamic preservation and sepsis‐like mitigation after asphyxial cardiac arrest. Crit Care Med. 2015;43:e420. [DOI] [PubMed] [Google Scholar]
- 9. Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003;34:2495–2501. [DOI] [PubMed] [Google Scholar]
- 10. Beurskens CJ, Horn J, de Boer AMT, Schultz MJ, van Leeuwen EM, Vroom MB, Juffermans NP. Cardiac arrest patients have an impaired immune response, which is not influenced by induced hypothermia. Crit Care. 2014;18:R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bro‐Jeppesen J, Kjaergaard J, Stammet P, Wise MP, Hovdenes J, Åneman A, Horn J, Devaux Y, Erlinge D, Gasche Y, et al.; TTM‐Trial Investigators . Predictive value of interleukin‐6 in post‐cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation. 2016;98:1–8. [DOI] [PubMed] [Google Scholar]
- 12. Vaahersalo J, Skrifvars MB, Pulkki K, Stridsberg M, Røsjø H, Hovilehto S, Tiainen M, Varpula T, Pettilä V, Ruokonen E, et al. Admission interleukin‐6 is associated with post resuscitation organ dysfunction and predicts long‐term neurological outcome after out‐of‐hospital ventricular fibrillation. Resuscitation. 2014;85:1573–1579. [DOI] [PubMed] [Google Scholar]
- 13. Tang YH, Vital S, Russell J, Seifert H, Granger DN. Interleukin‐6 mediates enhanced thrombus development in cerebral arterioles following a brief period of focal brain ischemia. Exp Neurol. 2015;271:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Sadowska GB, Chen X, Park SY, Kim J‐E, Bodge CA, Cummings E, Lim Y‐P, Makeyev O, Besio WG, et al. Anti–IL‐6 neutralizing antibody modulates blood‐brain barrier function in the ovine fetus. FASEB J. 2015;29:1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demené C, Maresca D, Kohlhauer M, Lidouren F, Micheau P, Ghaleh B, Pernot M, Tissier R, Tanter M. Multi‐parametric functional ultrasound imaging of cerebral hemodynamics in a cardiopulmonary resuscitation model. Sci Rep. 2018;8:16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugita A, Kinoshita K, Sakurai A, Chiba N, Yamaguchi J, Kuwana T, Sawada N, Hori S. Systemic impact on secondary brain aggravation due to ischemia/reperfusion injury in post‐cardiac arrest syndrome: a prospective observational study using high‐mobility group box 1 protein. Crit Care. 2017;21:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Omura T, Kushimoto S, Yamanouchi S, Kudo D, Miyagawa N. High‐mobility group box 1 is associated with neurological outcome in patients with post‐cardiac arrest syndrome after out‐of‐hospital cardiac arrest. J Intensive Care. 2016;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tissier R, Hocini H, Tchitchek N, Deye N, Legriel S, Pichon N, Daubin C, Hermine O, Carli P, Vivien B, et al. Early blood transcriptomic signature predicts patients’ outcome after out‐of‐hospital cardiac arrest. Resuscitation. 2019;138:222–232. [DOI] [PubMed] [Google Scholar]
- 19. Hachimi‐Idrissi S, Van Hemelrijck A, Michotte A, Smolders I, Sarre S, Ebinger G, Huyghens L, Michotte Y. Postischemic mild hypothermia reduces neurotransmitter release and astroglial cell proliferation during reperfusion after asphyxial cardiac arrest in rats. Brain Res. 2004;1019:217–225. [DOI] [PubMed] [Google Scholar]
- 20. Horiguchi T, Shimizu K, Ogino M, Suga S, Inamasu J, Kawase T. Postischemic hypothermia inhibits the generation of hydroxyl radical following transient forebrain ischemia in rats. J Neurotrauma. 2003;20:511–520. [DOI] [PubMed] [Google Scholar]
- 21. Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK. Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis. 2002;11:28–42. [DOI] [PubMed] [Google Scholar]
- 22. Schock RB, Janata A, Peacock WF, Deal NS, Kalra S, Sterz F. Time to cooling is associated with resuscitation outcomes. Ther Hypothermia Temp Manag. 2016;6:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanger D, Kawano T, Malhi N, Grunau B, Tallon J, Wong GC, Christenson J, Fordyce CB. Door‐to‐targeted temperature management initiation time and outcomes in out‐of‐hospital cardiac arrest: insights from the continuous chest compressions trial. J Am Heart Assoc. 2019;8:e012001 DOI: 10.1161/JAHA.119.012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adrie C, Adib‐Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh‐Xuan AT, Carli P, Spaulding C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis‐like” syndrome. Circulation. 2002;106:562–568. [DOI] [PubMed] [Google Scholar]
- 25. Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou J‐F, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis‐like syndrome? Curr Opin Crit Care. 2004;10:208–212. [DOI] [PubMed] [Google Scholar]
- 26. McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL‐6 trans‐signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA. 2005;102:9589–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park JS, Arcaroli J, Yum H‐K, Yang H, Wang H, Yang K‐Y, Choe K‐H, Strassheim D, Pitts TM, Tracey KJ, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–C879. [DOI] [PubMed] [Google Scholar]
- 28. Andersson U, Wang H, Palmblad K, Aveberger A‐C, Bloom O, Erlandsson‐Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (Hmg‐1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asavarut P, Zhao H, Gu J, Ma D. The role of HMGB1 in inflammation‐mediated organ injury. Acta Anaesthesiol Taiwan. 2013;51:28–33. [DOI] [PubMed] [Google Scholar]
- 30. Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL‐6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–175. [DOI] [PubMed] [Google Scholar]
- 31. Niemann JT, Rosborough JP, Youngquist S, Shah AP, Lewis RJ, Phan QT, Filler SG. Cardiac function and the proinflammatory cytokine response after recovery from cardiac arrest in swine. J Interferon Cytokine Res. 2009;29:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darbera L, Chenoune M, Lidouren F, Kohlhauer M, Adam C, Bruneval P, Ghaleh B, Dubois‐Randé J‐L, Carli P, Vivien B, et al. Hypothermic liquid ventilation prevents early hemodynamic dysfunction and cardiovascular mortality after coronary artery occlusion complicated by cardiac arrest in rabbits. Crit Care Med. 2013;41:e457–e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figures S1–S2


