Figure 4. Effect of proteolytic activity in urine from diabetic and plasminogen‐deficient (Plg−/−) mice on inward current in M1 cells and abundance of γ‐epithelial sodium channel (ENaC) and α‐ENaC subunits in kidney tissue.
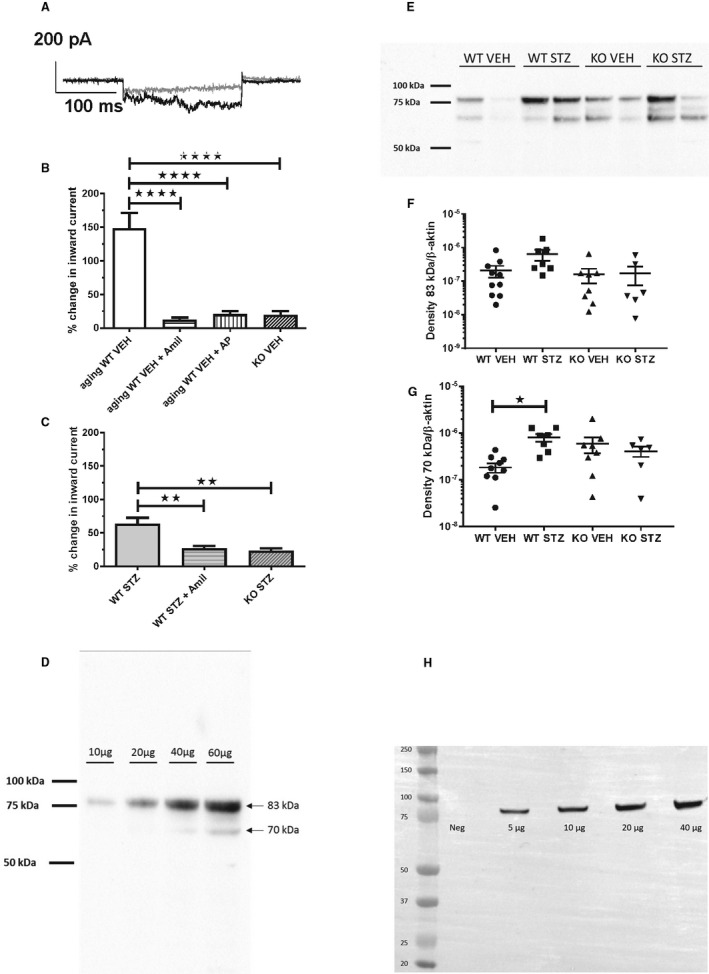
A, Patch‐clamp current trace of M1 cell before (gray line) and after (black line) addition of 24‐hour urine aliquot from a diabetic wild‐type (WT) mouse. B, Urine from aging WT vehicle mice (n=5 in all groups) significantly stimulated inward current. Pretreatment with amiloride (Amil, 2 μmol/L) or α2‐antiplasmin (AP, 1 μmol/L) abolished inward current evoked by urine. The response with urine from knockout vehicle mice was impaired compared with WT mice. ****P<0.0001. C, 24‐hour urine from WT streptozotocin mice stimulated significantly Amil‐sensitive inward current relative to knockout streptozotocin (n=5 in all groups). Urine samples from streptozotocin animals were dilute as a result of significant 5 to 6 times greater diuresis (Table S1). **P<0.01. (D‐H) Immunoblot analysis of kidney homogenates from the vehicle (VEH) and diabetic (STZ) treated wild‐type (WT) and Plg‐/‐ (KO) mice for ϒ‐ENaC (D) and α‐ENaC (H). SIze markers are shown in kilo Daltons (kDa). Concentration‐response with an increasing amount of homogenate protein shows a linear relation with densitometry for γ‐ENaC (D) and α‐ENaC (H). The predicted sizes of full‐length glycosylated proteins are 85‐90 kDa. E shows a representative blot for γ‐ENaC and F‐G shows the densitometric evaluation of full length protein migrating at 80‐85 kDa and proteolytic product at 65‐70 kDa. ∗ P≤0.05, n=10 (WT‐STZ), n=8 (KO VEH), n= 6 (KO‐STZ).
