Abstract
Background
Sleep fragmentation and sleep apnea are common in patients with atrial fibrillation (AF). We investigated the impact of radio‐frequency catheter ablation (RFCA) on sleep quality in patients with paroxysmal AF and the effect of a change in sleep quality on recurrence of AF.
Methods and Results
Of 445 patients who underwent RFCA for paroxysmal AF between October 2007 and January 2017, we analyzed 225 patients who had a 24‐hour Holter test within 6 months before RFCA. Sleep quality was assessed by cardiopulmonary coupling analysis using 24‐hour Holter data. We compared cardiopulmonary coupling parameters (high‐frequency coupling, low‐frequency coupling, very‐low‐frequency coupling) before and after RFCA. Six months after RFCA, the high‐frequency coupling (marker of stable sleep) and very‐low‐frequency coupling (rapid eye movement/wake marker) was significantly increased (29.84%–36.15%; P<0.001; and 26.20%–28.76%; P=0.002, respectively) while low‐frequency coupling (unstable sleep marker) was decreased (41.25%–32.13%; P<0.001). We divided patients into 3 tertiles according to sleep quality before RFCA, and the risk of AF recurrence in each group was compared. The second tertile was used as a reference; patients with unstable sleep (Tertile 3) had a significantly lower risk of AF recurrence (hazard ratio [HR], 0.32; 95% CI, 0.12–0.83 for high‐frequency coupling; and HR, 0.22; 95% CI, 0.09–0.58 for low‐frequency coupling).
Conclusions
Sleep quality improved after RFCA in patients with paroxysmal AF. The recurrence rate was significantly lower in patients who had unstable sleep before RFCA. These results suggest that RFCA can influence sleep quality, and sleep quality assessment before RFCA may provide a risk marker for recurrence after RFCA in patients with paroxysmal AF.
Keywords: 24‐hour Holter study, atrial fibrillation, cardiopulmonary coupling analysis, sleep disorders, sleep quality
Subject Categories: Arrhythmias, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- CPC
cardiopulmonary coupling
- EDR
ECG‐derived respiration
- e‐LFC
elevated‐low frequency coupling
- HFC
high‐frequency coupling
- LFC
low‐frequency coupling
- MrOS
Osteoporotic Fractures in Men Study
- RFCA
radio‐frequency catheter ablation
Clinical Perspective
What Is New?
Sleep quality estimated by an ECG‐based analysis was improved after radio‐frequency catheter ablation (RFCA) in patients with paroxysmal atrial fibrillation.
The recurrence rate of atrial fibrillation was significantly lower in patient who had unstable sleep before RFCA.
The worse the sleep quality before RFCA, the less likely the recurrence of atrial fibrillation; this could plausibly be the result of an improvement in sleep quality.
What Are the Clinical Implications?
RFCA has the additional benefit of improving sleep quality beyond rhythm control.
Sleep quality assessment before RFCA may provide a risk marker for recurrence after RFCA in patients with paroxysmal atrial fibrillation who undergo RFCA.
Atrial fibrillation (AF) is the most common arrhythmia in late life, and its prevalence has rapidly increased over the past decades. 1 It is associated with increased mortality, morbidity, and rising economic costs. 2 A substantial number of patients have recurrences despite initially successful radio‐frequency catheter ablation (RFCA) therapy. 3 There is a need to identify correctible or modifiable novel risk factors that can predict AF recurrence, in turn offering new intervention targets. The sleep state offers 2 such possibilities—sleep apnea and sleep quality/fragmentation.
A direct link between AF and sleep apnea is supported by clinical 4 and mechanistic data. 5 , 6 The severity of sleep apnea is associated with subclinical left atrial (LA) disease, as indicated by P wave terminal force in V1. 7 Obstructive sleep apnea is associated with significant atrial remodeling. 8 Obstructive sleep apnea increases the risk of new‐onset AF 9 as well as recurrence of AF after cardioversion or RFCA. 10 An association between central sleep apnea and AF is even more striking. 11 Epidemiological observations from the Sleep Heart Health Study 12 and MrOS (Osteoporotic Fractures in Men Study) 13 report incident AF with baseline central sleep apnea.
A relationship of sleep quality or quantity to AF has been less well developed. In the Multi‐Ethnic Study of Atherosclerosis, sleep quality was poor (reduced slow‐wave sleep) in those with AF. 14 In the Physicians' Health Study, shorter sleep duration was associated with a higher risk of AFn in those with prevalent sleep apnea, 15 while long sleep duration was related to the arrhythmia in a Chinese cohort. 16 Insomnia has also been linked to AF risk. 17 In a recent analysis across multiple cohorts (Health eHeart Study, the Cardiovascular Health Study, and the California Healthcare Cost and Utilization Project), sleep disruption consistently predicted AF before and after adjustment for obstructive sleep apnea and other potential confounders across several different populations. 18
Continuous electrocardiography is a clinical standard in the management of cardiac arrhythmias. Extracting sleep physiology and pathology information from the ECG recorded during the sleep period has been done in research for a long time but has typically focused on heart rate variability measures. There is increasing interest in computing sleep information from ECG signals, driven in part by developments in wearable technologies. The ECG‐based cardiopulmonary coupling (CPC) technique can generate measures of sleep quality (without an electroencephalogram) and surrogate measures of sleep apnea and central sleep apnea or periodic breathing (the sleep spectrogram). 19 , 20 , 21 CPC can be used as an ambulatory sleep quality marker to assess night‐to‐night dynamics or longer‐range treatment outcomes. 21 We used this approach to test the hypothesis that an ECG‐CPC spectrogram identified sleep quality, specifically impaired sleep quality, would be associated with an increased risk of AF recurrence in those undergoing RFCA.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Patient Population
Four hundred forty‐five patients who underwent RFCA for paroxysmal AF at Korea University Guro Hospital from October 2007 to January 2017 were selected for analysis (Figure 1). We analyzed 225 patients who had a 24‐hour Holter test within 6 months before RFCA to investigate the effect of sleep quality on AF recurrence. To investigate the impact of RFCA on sleep quality, we analyzed 218 patients who had a 24‐hour Holter test within 6 months before and after RFCA (Figure 1). In our center, a 24 hour‐Holter test is routinely performed 1, 3, 6 months, and 12 months after RFCA to evaluate the recurrence of AF. AF recurrence was assessed as the first documented atrial tachyarrhythmia lasting more than 30 seconds on 24 hour‐Holter test or the first documented atrial tachyarrhythmia on single‐strip 12‐lead surface electrocardiography after the 3‐month blanking period. This study was approved by the Institutional Review Board. The requirement for written informed consent was waived because of the retrospective design of the study. Demographics and echocardiographic data were extracted from the medical record.
Figure 1. Flow diagram illustrating patients with paroxysmal AF who were included in this study.
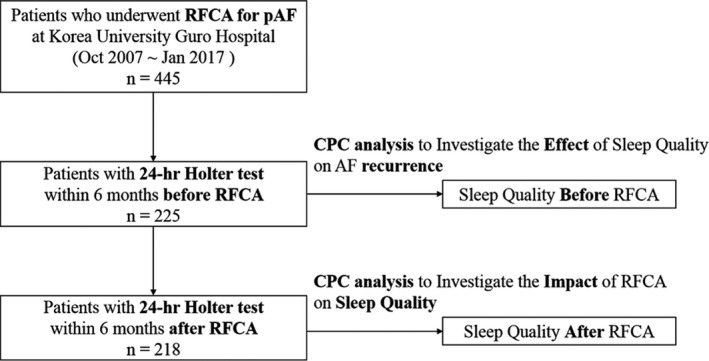
Four hundred forty‐five patients who underwent RFCA for paroxysmal AF were selected for analysis. Of those, 225 underwent a 24‐hour Holter test within 6 months before RFCA. Of the 225 patients, 218 underwent a 24‐hour Holter within 6 months before and after RFCA. The sleep quality changes after RFCA were the difference between CPC parameters before and after RFCA. AF indicates atrial fibrillation; CPC, cardiopulmonary coupling; and RFCA, radio‐frequency catheter ablation.
Data Management
Each patient's Holter results were converted to individual deidentified *.edf files and then imported into RemLogic V1.1. This version enables the CPC algorithm. Because Holter data were analyzed in a retrospective manner, there was no direct information about sleep onset and offset. Therefore, the time at which the heart rate began to stabilize was assumed to be the sleep onset time, and the sleep duration was assumed to be 7 hours.
Sleep State Analysis Strategy
The pre‐RFCA CPC parameters were obtained from the 24‐hour Holter performed within 6 months before RFCA. Importantly, the patients were in sinus rhythm at the time of recording. The sleep quality changes after RFCA were the differences between CPC parameters before and after RFCA. We also investigated the effect of sleep quality before RFCA on AF recurrence by analyzing the correlation between pre‐RFCA CPC parameters and AF recurrence.
CPC Analysis
CPC analysis was performed as described in the previous literature. 19 , 20 , 21 Briefly, information on heart rate variability and ECG‐derived respiration (EDR) were extracted and computed from the 24 hour‐Holter study. The EDR is the amplitude variations in the QRS complex. The time series of normal‐to‐normal sinus intervals and the time series of the EDR associated with these normal‐to‐normal sinus intervals are then extracted from the RR (QRS to QRS) interval time series. After removing abnormal signals attributable to false or missed R‐wave detections, resampling these 2 signals (normal‐to‐normal sinus interval series and its associated EDR) at 2 Hz using cubic splines is done. The cross‐spectral power and coherence of these signals are calculated over a 1024 sample window using the fast Fourier transform applied to the 3 overlapping 512 sample subwindows within the 1024 coherence window. The 1024 coherence window is then advanced by 256 samples and the calculation repeated until the analysis of the entire normal‐to‐normal sinus interval/EDR series was complete. The ratio of coherent cross power in the low‐frequency (0.01–0.1 Hz.) band to that in the high frequency (0.1–0.4 Hz.) band is calculated using the product of the coherence and cross‐spectral power for each 1024 window. This ratio is used to classify each sampling window as a high‐frequency coupling or low‐frequency coupling. When the low‐frequency coupling predominates, it suggests periodic respiration during the “unstable state,” but when the high‐frequency coupling predominates, it suggests physiologic respiratory sinus arrhythmia and “stable” state. Very‐low‐frequency coupling, which means wake or rapid eye movement sleep, was calculated using the ratio of coherent cross power in the 0‐ to 0.01‐Hz band to the power in the 0.01‐ to 0.4‐Hz band. In addition, previous studies have identified a subset of low‐frequency coupling called elevated low‐frequency coupling (e‐LFC). 19 , 20 , 21 The frequency band defining e‐LFC provides information on apnea/hypopnea. Narrow spectral band e‐LFC—periods with oscillations that have a single dominant coupling frequency—suggests central sleep apnea or periodic respiration. Meanwhile, broad spectral band e‐LFC—periods with oscillations that have variable coupling frequencies—suggests anatomic obstructive sleep apnea.
Statistical Analysis
Data are presented as means±SD or numbers and percentages. The comparisons between patients with AF recurrence and those without AF recurrence were tested by independent sample t test/Mann‐Whitney U test or chi‐square test as appropriate. CPC parameters before and after RFCA were compared using the paired t test. Criteria for sleep instability were not yet established, so patients were divided into 3 tertiles according to sleep parameters (HFC, stable sleep marker; LFC, unstable marker). The second tertile was used as a reference; the first tertile was defined as stable sleep, and the third tertile was defined as unstable sleep. Tertiles 1, 2, and 3 according to HFC (H1, H2, and H3) were defined as >36.7%, 21.2% to 36.7%, and <21.2%, respectively, and according to LFC (L1, L2, and L3) were defined as <34.2%, 34.2% to 48.2%, and >48.2%, respectively. The risk of AF recurrence in each group was compared using a Cox analysis after adjusting for multivariate variables. The analyses were conducted using SPSS Statistics 20.0 (IBM, Chicago, IL).
RESULTS
Baseline Characteristics
Of the 445 patients who underwent RFCA, we analyzed 225 patients who had a 24‐hour Holter test within 6 months before RFCA (Figure 1). The patient population consisted of 80 women (35.6%) and 145 men, with a mean age of 57.6±11 years. During a mean follow‐up period of 39.2±24.3 months, 36 patients (16%) experienced AF recurrences after RFCA. The average LA size in our study was 39.8±5.3 mm, and mean duration of AF was 451.8±48.0 days. Table 1 shows the baseline characteristics: overall and recurrence. Demographic and clinical variables were not significantly different between the 2 groups except LA volume. Patients who experienced AF recurrence had a larger LA volume (measured by computed tomography; P<0.05) and LA volume index (measured by echocardiography; P<0.005) than those who did not. There was no significant mean difference in baseline pre‐RFCA CPC parameters between the 2 groups.
Table 1.
Baseline Characteristics
| All (n=225) | Nonrecur (n=189) | Recur (n=36) | P Value | |
|---|---|---|---|---|
| Age, y | 57.63±10.96 | 57.32±11.24 | 59.22±9.39 | 0.342 |
| Weight, kg | 67.80±9.53 | 67.61±9.60 | 68.79±9.17 | 0.496 |
| Height, cm | 165.85±8.60 | 166.02±8.72 | 164.95±8.01 | 0.495 |
| BMI, kg/m2 | 24.61±2.69 | 24.49±2.61 | 25.28±3.01 | 0.105 |
| AF duration, d | 451.84±47.99 | 451.93±52.04 | 451.42±125.61 | 0.997 |
| CHA2DS2Vasc score | 1.57±1.29 | 1.54±1.29 | 1.75±1.30 | 0.370 |
| CHF, n (%) | 21 (9.3) | 16 (8.5) | 5 (13.9) | 0.305 |
| Hypertension, n (%) | 104 (46.2) | 87 (46.0) | 17 (47.2) | 0.896 |
| Age (>75 y) | 6 (2.7) | 5 (2.6) | 1 (2.8) | 0.964 |
| Diabetes mellitus, n (%) | 42 (18.7) | 35 (18.5) | 7 (19.4) | 0.896 |
| Stroke, n (%) | 14 (6.2) | 12 (6.3) | 2 (5.6) | 0.857 |
| TIA, n (%) | 5 (2.2) | 3 (1.6) | 2 (5.6) | 0.182 |
| Vascular disease, n (%) | 1 (0.4) | 1 (0.5) | 0 (0) | 0.662 |
| Age (65–75 y), n (%) | 56 (24.9) | 46 (24.3) | 10 (27.8) | 0.662 |
| Female, n (%) | 80 (35.6) | 66 (34.9) | 14 (38.9) | 0.648 |
| CT | ||||
| LA volume (CT), mL | 119.17±26.93 | 117.43±25.54 | 128.26±32.16 | 0.027 |
| LAA volume (CT), mL | 10.39±3.85 | 10.38±3.96 | 10.47±3.24 | 0.889 |
| RA volume (CT), mL | 117.22±26.08 | 117.04±27.23 | 118.18±19.26 | 0.811 |
| Pericardial fat, mL | 85.92±32.14 | 87.35±33.10 | 78.42±25.69 | 0.127 |
| Echocardiography | ||||
| LV ejection fraction, % | 66.62±7.43 | 66.33±7.50 | 68.11±7.02 | 0.190 |
| LA size, mm | 39.82±5.31 | 39.63±5.17 | 40.76±5.95 | 0.244 |
| LA volume index, mL/m2 | 32.36±14.78 | 30.91±13.60 | 39.71±18.22 | 0.001 |
| E/E' | 10.03±4.00 | 10.19±4.19 | 9.15±2.58 | 0.191 |
| Sleep parameter | ||||
| HFC, % | 29.67±16.74 | 29.07±17.00 | 32.81±15.15 | 0.221 |
| LFC, % | 41.28±14.05 | 41.50±14.49 | 40.16±11.54 | 0.600 |
| VLFC, % | 26.37±9.66 | 26.64±9.88 | 24.94±8.35 | 0.332 |
| NB, % | 2.05±4.02 | 2.03±4.13 | 2.15±3.45 | 0.874 |
| BB, % | 23.36±12.04 | 23.63±12.33 | 21.98±10.45 | 0.452 |
Values are presented as mean±SD or number (%). BB indicates broadband; BMI, body mass index; CHF, congestive heart failure; HFC, high‐frequency coupling; LA, left atrial; LAA, left atrial appendage; LFC, low‐frequency coupling; LV, left ventricular; NB, narrow band; RA, right atrial; TIA, transient ischemic attack; and VLFC, very‐low‐frequency coupling.
Sleep Quality Change After RFCA
Table 2 shows the changes in sleep quality after RFCA. All CPC parameters improved significantly after RFCA. Percentages of HFC and very‐low‐frequency coupling increased (29.84%–36.15%; P<0.001; and 26.20–28.76%; P=0.002, respectively) while the percentage of LFC, the marker of unstable sleep, decreased (41.25%–32.13%; P<0.001) after RFCA. The percentage of narrow and broad band also decreased significantly after RFCA (2.09%–1.36%; P=0.009; and 23.31%–18.58%; P<0.001, respectively). Figure 2 shows an example of CPC analysis, which shows dramatic changes in sleep quality before and after RFCA.
Table 2.
Sleep Quality Change After RFCA in Paroxysmal Atrial Fibrillation
| Pre‐RFCA (n=218) | Post‐RFCA (n=218) | Difference (95% CI) | P Value | |
|---|---|---|---|---|
| HFC, % | 29.84±16.62 | 36.15±18.09 | 6.31±18.58 (3.83 to 8.79) | <0.001 |
| LFC, % | 41.25±14.06 | 32.13±15.69 | −9.12±16.40 (−11.30 to −6.93) | <0.001 |
| VLFC, % | 26.20±9.65 | 28.76±10.90 | 2.56±12.29 (0.92 to 4.20) | 0.002 |
| NB, % | 2.09±4.077 | 1.36±2.94 | −0.73±4.06 (−1.27 to −0.18) | 0.009 |
| BB, % | 23.31±12.06 | 18.58±12.27 | −4.73±12.83 (−6.44 to −3.02) | <0.001 |
Values are presented as mean±SD. BB indicates broadband; HFC, high‐frequency coupling; LFC, low‐frequency coupling; NB, narrow band; RFCA, radio‐frequency catheter ablation; and VLFC, very‐low‐frequency coupling.
Figure 2. The ECG‐derived sleep spectrogram.
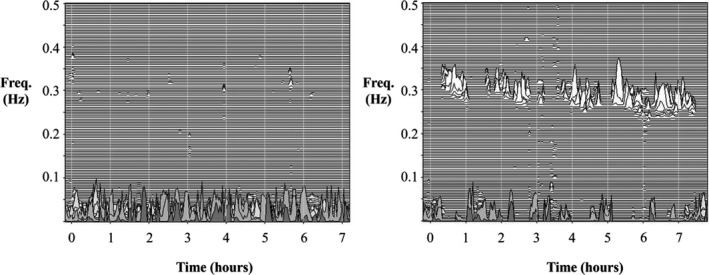
The left part of the figure shows absence of stable sleep, with sleep state dominated by low‐frequency cardiopulmonary coupling. The right part of the figure is the sleep spectrogram 6 months after RFCA. It shows a marked return of stable sleep dominated by high‐frequency cardiopulmonary coupling. This patient did not recur during the follow‐up period. RFCA indicates radio‐frequency catheter ablation.
Effect of Sleep Quality on Recurrence of AF
The risk of AF recurrence according to sleep status is presented in Figure 3. When the sleep quality was classified according to HFC, patients in the H3 group (unstable sleep, HFC <21.2%) had a significantly lower risk of AF recurrence compared with the reference group (hazard ratio [HR], 0.32; 95% CI, 0.12–0.83) (Figure 3A). This trend was consistent even when classified according to LFC. Patients in the L3 group (unstable sleep, LFC >48.2%) had a lower risk of recurrence than in the reference group (HR, 0.22; 95% CI, 0.09–0.58) (Figure 3B). However, patients with stable sleep (H1 and L1) did not have a significantly increased or decreased risk of recurrence compared with the reference group. The baseline characteristics according to sleep status are compared in Tables S1 and S2.
Figure 3. Sleep status and adjusted hazard ratios for AF recurrence.
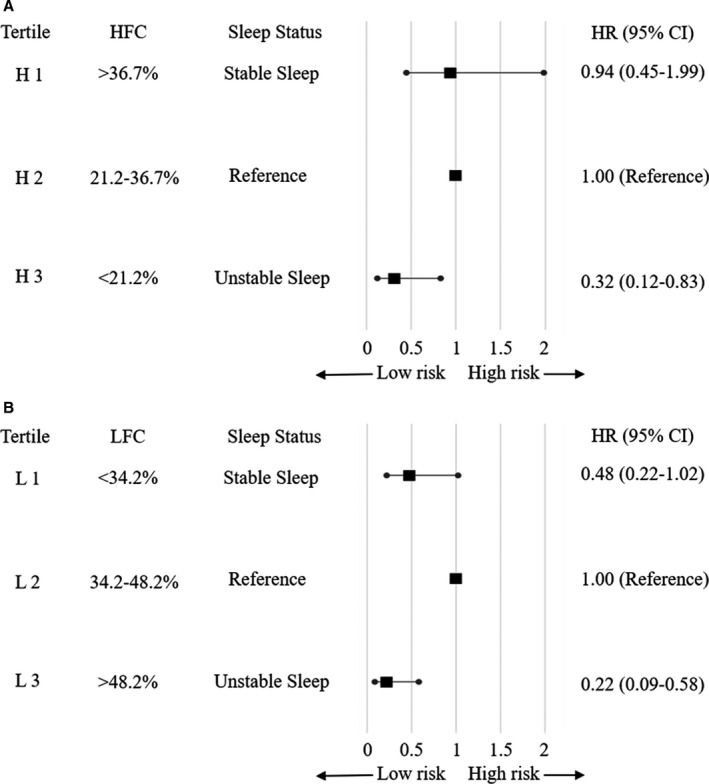
A, Sleep status according to the HFC tertile; (B) Sleep status according to the LFC tertile. The subjects were divided into tertiles according to sleep quality, and the risk of AF recurrence in each group was compared. The second tertile was used as a reference, the first tertile was defined as stable sleep, and the third tertile was defined as unstable sleep. Tertiles 1, 2, and 3 according to HFC (H1, H2, and H3) were defined as >36.7%, 21.2% to 36.7%, and <21.2%, respectively, and according to LFC (L1, L2, and L3) were defined as <34.2%, 34.2% to 48.2%, and >48.2%, respectively. Adjusted for age, sex, body mass index, AF duration, diabetes mellitus, hypertension, left atrial volume index, left ventricular ejection fraction, and creatinine. AF indicates atrial fibrillation; HFC, high‐frequency coupling; HR, hazard ratio; and LFC, low‐frequency coupling.
Sleep State Parameters: Recurrence Versus No Recurrence
The quality of sleep after RFCA was significantly improved in patients without AF recurrence, but sleep quality changes were relatively small in patients with AF recurrence (Tables 3 and 4).
Table 3.
Subgroup Analysis of Sleep Quality Change After RFCA: Nonrecur
| Pre‐RFCA | Post‐RFCA | Difference (95% CI) | P Value | |
|---|---|---|---|---|
| HFC% | 29.25±16.87 | 36.05±17.91 | 6.80±18.80 (4.05 to 9.55) | <0.001 |
| LFC% | 41.46±14.52 | 31.93±15.17 | −9.53±16.18 (−11.90 to −7.16) | <0.001 |
| VLFC% | 26.45±9.89 | 29.04±10.81 | −2.58±12.64 (−4.43 to −0.74) | 0.006 |
| NB% | 2.08±4.20 | 1.25±2.61 | 0.83±3.77 (0.27 to 1.38) | 0.004 |
| BB% | 23.57±12.37 | 18.49±11.89 | 5.08±12.86 (3.20 to 6.96) | <0.001 |
BB indicates broadband; HFC, high‐frequency coupling; LFC, low‐frequency coupling; NB, narrow band; and RFCA, radio‐frequency catheter ablation.
Table 4.
Subgroup Analysis of Sleep Quality Change After RFCA: Recur
| Pre‐RFCA | Post‐RFCA | Difference (95% CI) | P Value | |
|---|---|---|---|---|
| HFC% | 32.81±15.15 | 36.63±19.22 | 3.82±17.50 (−9.74 to +2.10) | 0.199 |
| LFC% | 40.16±11.54 | 33.14±18.30 | −7.02±17.55 (−12.95 to −1.08) | 0.022 |
| VLFC% | 24.94±8.35 | 27.37±11.38 | −2.43±10.51 (−5.99 to 1.13) | 0.174 |
| NB% | 2.15±3.45 | 1.93±4.26 | 0.22±5.34 (−1.59 to 2.03) | 0.807 |
| BB% | 21.98±10.45 | 19.03±14.23 | 2.95±12.71 (−1.35 to 7.25) | 0.173 |
BB indicates broadband; HFC, high‐frequency coupling; LFC, low‐frequency coupling; NB, narrow band; and RFCA, radio‐frequency catheter ablation.
DISCUSSION
The key findings of our study are as follows: (1) Sleep quality improved after RFCA in patients with paroxysmal AF; (2) improvement of sleep quality was particularly pronounced in patients without AF recurrence; and (3) the recurrence rate was significantly lower in patients who had unstable sleep before RFCA. That is, the worse the sleep before RFCA, the less likely the recurrence, plausibly from an improvement in sleep quality. In other words, if sleep quality improved after RFCA, the probability of recurrence was reduced. If sleep quality was already good before RFCA, improvements of sleep quality were unlikely and not likely to impact AF recurrences.
The results were contrary to our initial hypothesis, that worse sleep would predict increased recurrences. However, the change in sleep quality, which may be driven by both apnea and nonapnea mechanisms, may be the protective factor. Improved sleep quality can have substantial protective effects on cardiac arrhythmogenesis by reducing arousal‐related sympathetic effects and adverse effects of transient wakefulness episodes. 22 Secondary mechanisms such as improved blood pressure dipping may also contribute. 23
How may ablation improve sleep quality? One mechanism is by improving cardiac function and reducing pulmonary capillary wedge pressure, which would in turn reduce the propensity for respiratory instability and reduce sleep fragmentation. 24 , 25 The reported effects of AF treatment on overt sleep apnea have been inconsistent and largely negative 26 , 27 but may have played a role. The nucleus of the tractus solitarius is a key visceral integration center and closely communicates with nearby sleep and arousal centers, 28 such as the parabrachial nucleus, 29 , 30 which strongly modulates arousals from sleep. It is plausible that distortion of cardiac afferent signals from AF causes changes in parabrachial firing, though there are as yet no direct published data supporting the hypothesis.
This study has several clinical implications. Our data suggest that RFCA has an additional benefit of improving sleep quality beyond rhythm control, at least when quality is impaired at baseline. We also show a potential value for evaluating sleep quality before RFCA, and raise the possibility that improving sleep quality by other measures including pharmacological methods or sleep hygiene could have benefits. To our knowledge, this is the first study that investigates the impact of cardiac intervention like RFCA on sleep quality as well as the effect of sleep quality on recurrence of cardiac disease. Based on this study, the convenience of CPC analysis can be extended to investigate the relationship between sleep quality/instability and cardiac disease such as heart failure and coronary artery disease beyond AF.
Though we did not perform conventional polysomnography, there is substantial support for the use of the ECG‐CPC analysis as a measure of sleep quality. The amount of HFC is reduced by processes that fragment sleep such as sleep apnea. 31 ECG‐CPC has been shown to capture treatment effects in sleep apnea 32 and insomnia. 33 ECG‐derived sleep quality metrics examined to date show (1) independence of absolute EEG amplitudes and thus lack of constraint by “loss” of slow wave sleep with age 19 ; (2) associations with EEG‐defined stable and unstable sleep (cycling alternating pattern and noncycling alternating pattern) 19 such that HFC correlates with noncycling alternating pattern/stable sleep; (3) blood pressure dipping occurs only during periods of electroencephalogram–noncycling alternating pattern 34 ; (4) sleep spectrogram variables show heritability 35 ; (5) narrow spectral band e‐LFC is associated with hypertension and stroke 31 ; (6) HFC sleep is reduced in depression, 36 heart failure, 37 and fibromyalgia 38 ; (7) HFC is an independent determinant of the glucose disposition index 39 ; (8) pre/post treatment effects in sleep apnea are captured via changes in HFC/LFC. 40
This study has several limitations. Since this study was conducted in a single center, selection bias is somewhat inevitable. Compared with other studies, there was less recurrence in our study. Because the LA size of the subjects included in our study was small and the AF duration was relatively short, they were expected to have relatively less recurrence compared with other studies. Also, AF recurrence may be underestimated because of incomplete capture. However, despite the low recurrence, it is very encouraging that the effect of sleep status on recurrence is remarkable. Second, we do not have details from a comprehensive clinical sleep evaluation. Conventional polysomnography was not performed, so exact measures of sleep apnea (which was likely present in a subset) and standard sleep stages are not available. Because a 24‐hour Holter test was not performed to analyze sleep, our estimates of the sleep period are approximate. Unstable sleep can be induced by a variety of sleep‐disruptive conditions and reflects an integrated/composite output of the analysis algorithm—thus, contributions from sleep apnea and apnea‐independent fragmentation cannot be differentiated from our analysis. However, broad spectral band e‐LFC is most clearly linked to sleep apnea–driven cardiopulmonary oscillations, and thus contributions from apnea are likely. Finally, since criteria for sleep instability has not yet been established, sleep status is divided into tertiles according to HFC or LFC in our study. The inability to test and validate accurate and meaningful cutoff for stable/unstable sleep is a limitation of our study. However, we suggested the possibility that the sleep instability could predict recurrence and our findings could be the basis for research to find and validate the accurate cutoff in the future. A well‐designed prospective study is warranted to confirm these promising findings.
In conclusion, sleep quality as estimated by an ECG‐based analysis was improved after RFCA in patients with paroxysmal AF. The recurrence rate was significantly lower after RFCA in patients who had pre‐RFCA sleep instability and thus improvement post‐RFCA. These results suggest that RFCA can influence sleep quality, and sleep quality assessment before/after RFCA may provide a risk marker for recurrence after RFCA in patients with paroxysmal AF. Our results support the idea that manipulation of the sleep state could have benefits for AF recurrence, though such a premise needs to be tested directly through a randomized trial.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S2
(J Am Heart Assoc. 2020;9:e017016 DOI: 10.1161/JAHA.120.017016.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017016
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Jin Oh Na, Email: koolup93@gmail.com.
Robert J. Thomas, Email: rthomas1@bidmc.harvard.edu.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the united states. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 3. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts‐Thomson KC, Sanders P. Long‐term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004549 DOI: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. [DOI] [PubMed] [Google Scholar]
- 5. Yu L, Li X, Huang B, Zhou X, Wang M, Zhou L, Meng G, Wang Y, Wang Z, Deng J, et al. Atrial fibrillation in acute obstructive sleep apnea: autonomic nervous mechanism and modulation. J Am Heart Assoc. 2017;6:e006264 DOI: 10.1161/JAHA.117.006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos P, Rubies C, Torres M, Batlle M, Farre R, Brugada J, Montserrat JM, Almendros I, Mont L. Atrial fibrosis in a chronic murine model of obstructive sleep apnea: mechanisms and prevention by mesenchymal stem cells. Respir Res. 2014;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwon Y, Misialek JR, Duprez D, Alonso A, Jacobs DR Jr, Heckbert SR, Redline S, Soliman EZ. Association between sleep disordered breathing and electrocardiographic markers of atrial abnormalities: the MESA study. Europace. 2017;19:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, Antic N, Thornton A, Saint DA, McEvoy D, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–327. [DOI] [PubMed] [Google Scholar]
- 9. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 10. Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta‐analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108:47–51. [DOI] [PubMed] [Google Scholar]
- 11. Leung RS, Huber MA, Rogge T, Maimon N, Chiu KL, Bradley TD. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28:1543–1546. [DOI] [PubMed] [Google Scholar]
- 12. Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ, Rueschman M, Punjabi NM, Mehra R, Bertisch S, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc. 2017;6:e004500 DOI: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. May AM, Blackwell T, Stone PH, Stone KL, Cawthon PM, Sauer WH, Varosy PD, Redline S, Mehra R; Mr OSSSG . Central sleep‐disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med. 2016;193:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwon Y, Gharib SA, Biggs ML, Jacobs DR Jr, Alonso A, Duprez D, Lima J, Lin GM, Soliman EZ, Mehra R, et al. Association of sleep characteristics with atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis. Thorax. 2015;70:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khawaja O, Sarwar A, Albert CM, Gaziano JM, Djousse L. Sleep duration and risk of atrial fibrillation (from the Physicians’ Health Study). Am J Cardiol. 2013;111:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song Q, Liu X, Hu W, Zhou W, Liu A, Wang X, Wu S. Long sleep duration is an independent risk factor for incident atrial fibrillation in a Chinese population: a prospective cohort study. Sci Rep. 2017;7:3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee HH, Chen YC, Chen JJ, Lo SH, Guo YL, Hu HY. Insomnia and the risk of atrial fibrillation: a population‐based cohort study. Acta Cardiol Sin. 2018;34:193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christensen MA, Dixit S, Dewland TA, Whitman IR, Nah G, Vittinghoff E, Mukamal KJ, Redline S, Robbins JA, Newman AB. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram‐based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151–1161. [DOI] [PubMed] [Google Scholar]
- 20. Thomas RJ, Mietus JE, Peng C‐K, Gilmartin G, Daly RW, Goldberger AL, Gottlieb DJ. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram‐based method. Sleep. 2007;30:1756–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas RJ, Wood C, Bianchi MT. Cardiopulmonary coupling spectrogram as an ambulatory clinical biomarker of sleep stability and quality in health, sleep apnea, and insomnia. Sleep. 2018;41:zsx196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uyama H, Yamauchi M, Fujita Y, Yoshikawa M, Ohnishi Y, Kimura H. The effects of arousal accompanying an apneic event on blood pressure and sympathetic nerve activity in severe obstructive sleep apnea. Sleep Breath. 2018;22:149–155. [DOI] [PubMed] [Google Scholar]
- 23. Javaheri S, Redline S. Sleep, slow‐wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14:442–448. [DOI] [PubMed] [Google Scholar]
- 24. Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea‐hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–1250. [DOI] [PubMed] [Google Scholar]
- 25. Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. [DOI] [PubMed] [Google Scholar]
- 26. Fox H, Bitter T, Horstkotte D, Oldenburg O. Cardioversion of atrial fibrillation or atrial flutter into sinus rhythm reduces nocturnal central respiratory events and unmasks obstructive sleep apnoea. Clin Res Cardiol. 2016;105:451–459. [DOI] [PubMed] [Google Scholar]
- 27. Hoglund N, Sahlin C, Kesek M, Jensen SM, Franklin KA. Cardioversion of atrial fibrillation does not affect obstructive sleep apnea. Ups J Med Sci. 2017;122:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cutsforth‐Gregory JK, Benarroch EE. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology. 2017;88:1187–1196. [DOI] [PubMed] [Google Scholar]
- 29. Qiu MH, Chen MC, Fuller PM, Lu J. Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra‐thalamic forebrain circuit nodes. Curr Biol. 2016;26:2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saper CB, Kaur S. Brain circuitry for arousal from apnea. Cold Spring Harb Symp Quant Biol. 2018;83:63–69. [DOI] [PubMed] [Google Scholar]
- 31. Thomas RJ, Weiss MD, Mietus JE, Peng CK, Goldberger AL, Gottlieb DJ. Prevalent hypertension and stroke in the Sleep Heart Health Study: association with an ECG‐derived spectrographic marker of cardiopulmonary coupling. Sleep. 2009;32:897–904. [PMC free article] [PubMed] [Google Scholar]
- 32. Choi JH, Thomas RJ, Suh SY, Park IH, Kim TH, Lee SH, Lee HM, Yun CH, Lee SH. Sleep quality change after upper airway surgery in obstructive sleep apnea: electrocardiogram‐based cardiopulmonary coupling analysis. Laryngoscope. 2015;125:1737–1742. [DOI] [PubMed] [Google Scholar]
- 33. Schramm PJ, Zobel I, Monch K, Schramm E, Michalak J. Sleep quality changes in chronically depressed patients treated with mindfulness‐based cognitive therapy or the cognitive behavioral analysis system of psychotherapy: a pilot study. Sleep Med. 2016;17:57–63. [DOI] [PubMed] [Google Scholar]
- 34. Iellamo F, Placidi F, Marciani MG, Romigi A, Tombini M, Aquilani S, Massaro M, Galante A, Legramante JM. Baroreflex buffering of sympathetic activation during sleep: evidence from autonomic assessment of sleep macroarchitecture and microarchitecture. Hypertension. 2004;43:814–819. [DOI] [PubMed] [Google Scholar]
- 35. Ibrahim LH, Jacono FJ, Patel SR, Thomas RJ, Larkin EK, Mietus JE, Peng CK, Goldberger AL, Redline S. Heritability of abnormalities in cardiopulmonary coupling in sleep apnea: use of an electrocardiogram‐based technique. Sleep. 2010;33:643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang AC, Yang CH, Hong CJ, Tsai SJ, Kuo CH, Peng CK, Mietus JE, Goldberger AL, Thomas RJ. Sleep state instabilities in major depressive disorder: detection and quantification with electrocardiogram‐based cardiopulmonary coupling analysis. Psychophysiology. 2011;48:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeh GY, Mietus JE, Peng CK, Phillips RS, Davis RB, Wayne PM, Goldberger AL, Thomas RJ. Enhancement of sleep stability with Tai Chi exercise in chronic heart failure: preliminary findings using an ECG‐based spectrogram method. Sleep Med. 2008;9:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas RJ, Mietus JE, Peng CK, Goldberger AL, Crofford LJ, Chervin RD. Impaired sleep quality in fibromyalgia: detection and quantification with ECG‐based cardiopulmonary coupling spectrograms. Sleep Med. 2010;11:497–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pogach MS, Punjabi NM, Thomas N, Thomas RJ. Electrocardiogram‐based sleep spectrogram measures of sleep stability and glucose disposal in sleep disordered breathing. Sleep. 2012;35:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee WH, Ahn J‐C, We J, Rhee C‐S, Lee CH, Yun P‐Y, Yoon I‐Y, Kim J‐W. Cardiopulmonary coupling analysis: changes before and after treatment with a mandibular advancement device. Sleep Breath. 2014;18:891–896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


