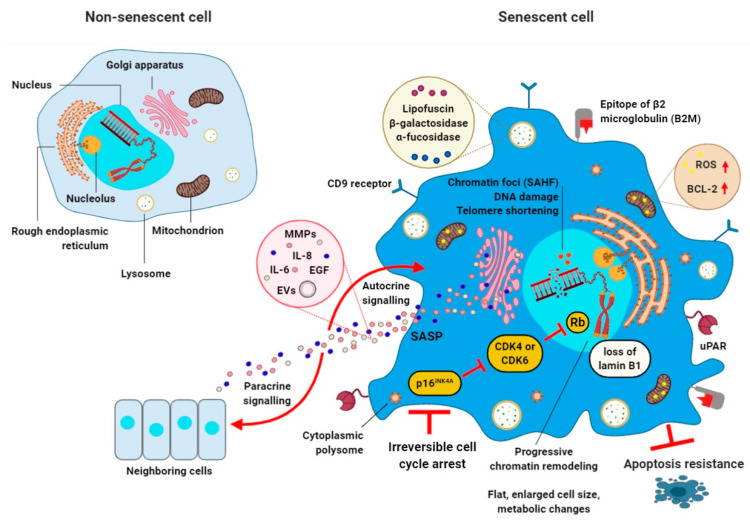
Figure 1.
Biomarkers of cellular senescence. Senescent cell (right) is characterized by irreversible cell cycle arrest induced by various stressors (e.g., DNA damage or telomere shortening). Classical features of senescent cells include flat and enlarged cell size, elevated expression of cell cycle inhibitors (such as p16INK4A or p21Cip1), increased nucleus size and multiple nucleoli, lysosomes overexpressing β-galactosidase, α-fucosidase, and lipofuscin, chromatin reorganization based on senescence-associated heterochromatic foci (SAHF) and loss of lamin B1, senescence-associated ribosome biogenesis defects (e.g., “free” cytoplasmic polysomes with ribosomes), the overexpression of anti-apoptotic Bcl-2 family members, reactive oxygen species (ROS) production in mitochondria, and expression of cell surface markers such as CD9 receptor, urokinase-type plasminogen activator receptor (uPAR), and epitope of β2 microglobulin (B2M). A characteristic key feature of cellular senescence is also the manifestation of the senescence-associated secretory phenotype (SASP) via the Golgi apparatus. SASP mediates the autocrine/paracrine activities of senescent cells by the secretion of matrix metalloproteinases (MMPs) such as MMP-3 and MMP-9, growth factors (e.g., epidermal growth factor (EGF)), cytokines and chemokines (e.g., IL-6 and IL-8), miRNAs, activins, and inhibins, lipids (e.g., ceramides), as well as exosome-like small extracellular vesicles (EVs). SASP can modify the microenvironment of senescent cells (SCs) and directly affects neighboring cells. A non-senescent cell is also presented (left).