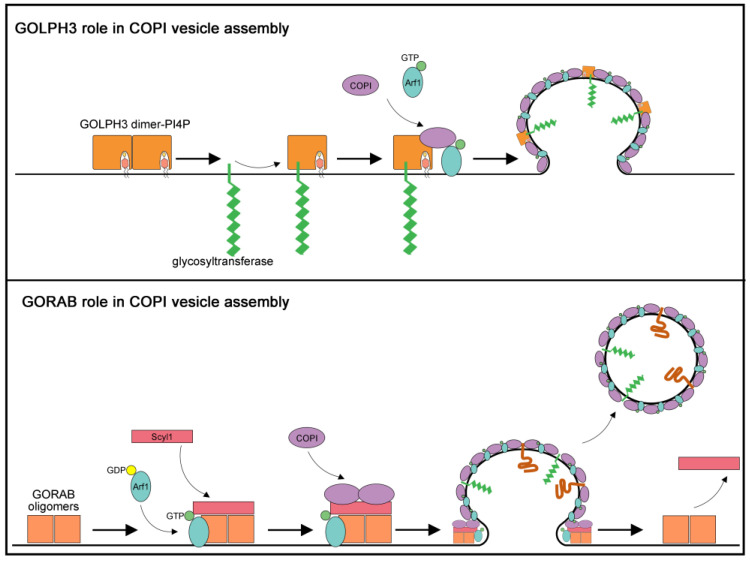
Figure 3.
Proposed models for roles of GORAB and GOLPH3 in COPI-mediated trafficking. (Upper panel) The diagram depicts a model for GOLPH3 role in N-glycosylation based on the results of Eckert and coauthors [65]. GOLPH3 protein binds to the coatomer as well as to a set of glycosyltransferases, such as C2GnT and SiaT as well as the coatomer, thereby mediating incorporation of these enzymes into COPI coated vesicles. (Lower panel) The diagram depicts a model for GORAB role in COPI vesicle release based on the results of Witkos and coauthors [66] GORAB self-associates in oligomers that form discrete domains at the trans-Golgi membrane. GORAB oligomers recruit Scyl1 protein driving Arf1-GTP accumulation in the GORAB-enriched domains. High levels of Scyl1 and Arf1-GTP proteins lead to the selective COPI loading. In turn, coatomer assembly leads to vesicle budding and cargo incorporation while GORAB/Scyl1 complex stabilizes the bud neck during vesicle formation. After releasing of the COPI vesicle from the Golgi membrane, GORAB and Scyl1 detach from the complex to start a new cycle.