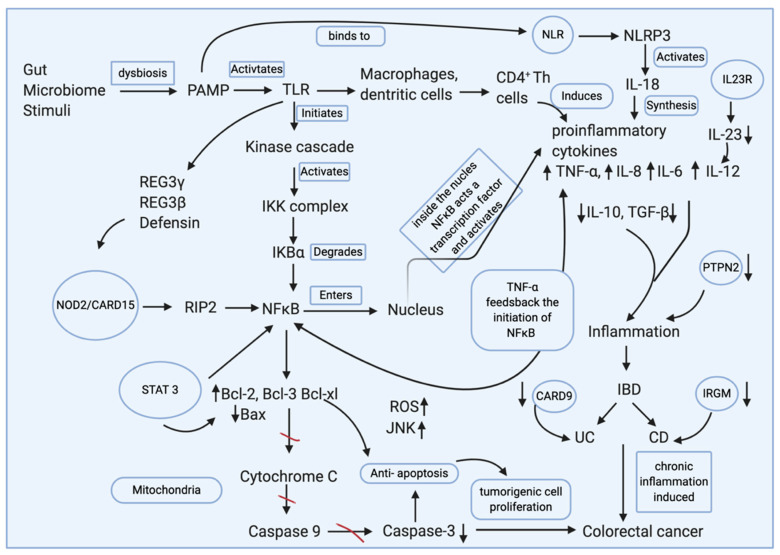
Figure 2.
Various gut microbial stimuli due to dysbiosis can cause the initiation of inflammation in the intestine. The release of the proinflammatory cytokines inducing inflammation through various pathways assures the destruction of pathogenic microbes, and homeostasis is maintained when the resolution phase is used for controlling the inflammation and damage repair. Dysbiosis, combined with genetic defects, can omit the resolution phase with continuous chronic inflammation leading to the introduction of IBD. The pathogen-associated molecular pattern (PAMP) releases can activate the Toll-like receptors (TLRs) to initiate the regenerating islet-derived protein (REG)3, REG3 and defensin that promotes the nucleotide-binding oligomerization domain-containing protein (NOD2) to release nuclear factor kappa B (NFB). A PAMP-activated TLR can also instigate the canonical pathway for the release of NFB through the activation of the kinase cascade that in turn activates the IKB kinase (IKK) complex where the degradation of IKK can release NFB into the nuclease. The NFB can act as a transcription factor in the production of various proinflammatory cytokines like tumor necrosis factor (TNF)-, interleukin (IL)-8, and IL-6. TNF- can provide feedback for the further initiation of NFB. PAMP can also bind to the nod-like receptor (NLR) gene, the NLR family pyrin domain containing 3 (NLRP3) that activates IL-18 that can synthesize other cytokines. A defect in the IL23R gene is responsible for the depletion of IL-23 that can be involved in the upregulation of IL-12 that stimulates inflammation. Chronic inflammation can lead to colorectal cancer, which is also influenced by the activation of NFB. The signal transducer and activator of transcription 3 (STAT3), along with NFB, can upregulate the antiapoptotic proteins B-cell lymphoma (Bcl)-2, Bcl-3, and B-cell lymphoma-extra-large (Bcl-xl), and it can diminish the apoptotic Bcl-2 associated X protein (Bax). This can irregulate caspase activity, thereby increasing the tumorigenic proliferation of susceptible cells developing into colorectal cancer. Image developed using Biorender.com.