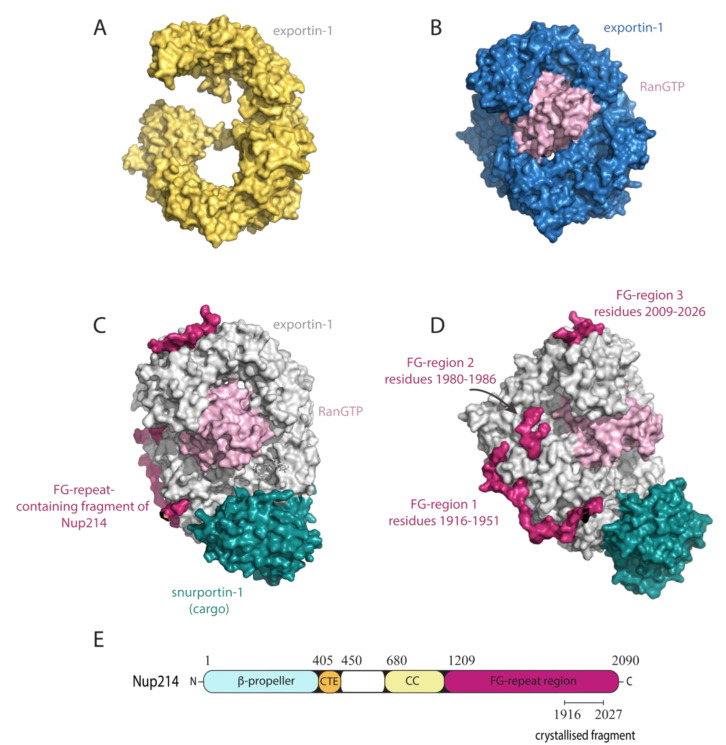
Figure 3.
The nuclear export receptor exportin-1 adopts an extended or compact conformation and can bind to multiple partners. (A–C) Surface representations of exportin-1 depicted in the same orientation. (A) The extended conformation of unbound exportin-1 from fungus. Figure generated from Protein Data Bank (PDB) ID 4FGV [54]. (B) The compact ring-like conformation of mouse exportin-1 (blue) bound to RanGTP (pink). Figure generated from Protein Data Bank (PDB) ID 3NC1 [58]. (C,D) The human exportin-1-RanGTP-snurportin-1-Nup214 complex, where D is a rotation of C along the Y-axis. RanGTP (pink) interacts with the inner surface of exportin-1 (grey). The nuclear export signal (NES) of the transport cargo, in this case snurportin-1 (teal), interacts with the NES binding groove on the outer surface of exportin-1. The receptor-cargo complex is transported through the nuclear pore complex (NPC) via its interactions with FG-Nups, such as Nup214 (crimson), of which a fragment (residues 1916-2026) containing three FG-regions (FG-region 1-3) is shown. Each of the three FG-regions contain multiple FG-repeats and serve as anchor points for binding hydrophobic surface pockets of exportin-1. Residues connecting the FG-repeat regions were not always clearly defined in the electron density. Although the Nup214 fragment was crystallised with an N-terminal maltose binding protein (MBP) fusion tag, MBP has been removed from the figure for clarity. Figure generated from PDB ID 5DIS [27]. (E) Schematic representation of the domain architecture of Nup214, with the crystallised fragment that is shown in C,D depicted. CTE = C-terminal extension of the seven-bladed β-propeller; CC = coiled-coil.