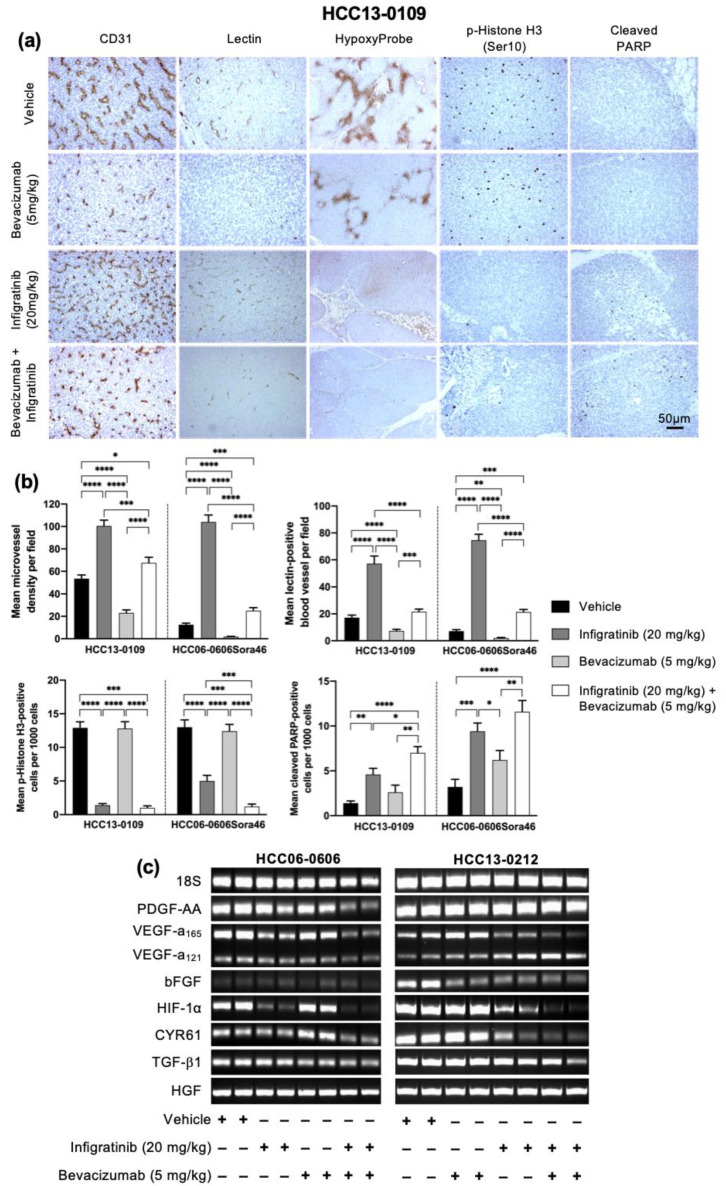
Figure 2.
Effects of Infigratinib/Bevacizumab on angiogenesis, vessel normalization, tumor hypoxia, cell proliferation, apoptosis, and expression of angiogenic factors in HCC models. Mice bearing HCC13-0109 xenografts were treated with vehicle, Infigratinib, Bevacizumab, or Infigratinib/Bevacizumab for 14 days, as described in Figure 1. Vehicle- and drug-treated mice were perfused with biotinylated lectin and injected with pimonidazole hydrochloride as described [23]. Tumors collected 2 h after the last treatments were processed for immunohistochemistry. Representative pictures of blood vessels stained with anti-CD31, proliferative cells stained with anti-p-histone H3 Ser10, cell death stained with anti-cleaved-PARP, functional blood vessels stained with lectin and tumor hypoxia stained with HypoxyProbe antibodies are shown (a). Bar: 50 μm. The number of p-Histone H3 Ser10 and cleaved PARP-positive cells, among at least 500 cells counted per region was determined and plotted as the mean number of positive cells per 1000 cells ± SE. The mean lectin-positive blood vessels and microvessel density ± SE from five random fields at a magnification of ×100 was also quantified. The difference in staining-positive cells were compared using Student’s t-test (b). RNA extractions were performed according to the Qiagen RNeasy protocol. The levels of vascular endothelial growth factor (VEGF), PDGF-AA, bFGF, CYR61, TGF-β1, HIF-1α and HGF mRNA were determined using the ViiA7 Real-time PCR system. Representative ethidium bromide-stained gels are shown (c). The primers used for RT-PCR are listed in Table 2 (Materials and Methods). Experiments were repeated twice with similar results. * p < 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001.