Table 6.
Structures and calculated free binding energies (∆GB, in kcal/mol) of the fifteen selected MNPs, one from in-house MNPs (hydroxydebromomarinone), two from MNPs pharmaceutical pipeline (nelarabine and fludarabine), and the positive (nelfinavir and lopinavir) and negative (allicin) controls, using two sets of search space coordinates.
| Code | Chemical Structure | Structural Category | Natural Source | Prob_A | ∆GB (kcal/mol) |
|---|---|---|---|---|---|
| 22947654 1 |
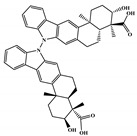
|
carbazole | marine derived bacteria | 0.42 | −9.9 6/−7.6 7 |
| 22947655 1 |
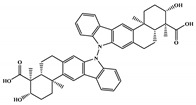
|
carbazole | marine derived bacteria | 0.42 | −9.9 6/−7.6 7 |
| 22435742 1 |
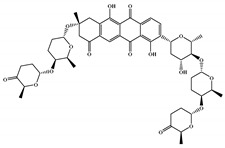
|
anthraquinone | marine derived bacteria | 0.42 | −9.4 6/−7.8 7 |
| 22435744 1 |
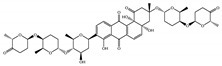
|
anthraquinone | marine derived bacteria | 0.41 | −9.4 6/−7.8 7 |
| 30380251 1 |
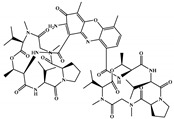
|
phenoxazinone | marine derived bacteria | 0.68 | −9.1 6/−6.9 7 |
| 19600610 1 |
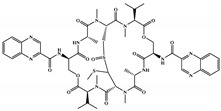
|
quinoxaline | marine derived bacteria | 0.62 | −8.9 6/−8.9 7 |
| 22435741 1 |
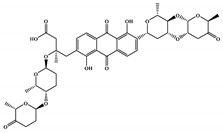
|
anthraquinone | marine derived bacteria | 0.40 | −8.8 6/−7.8 7 |
| 7450892 1 |
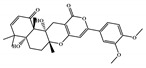
|
benzo[f]pyrano[4,3-b]chromene | marine derived fungus |
0.41 | −8.4 6/−6.9 7 |
| 19384758 1 |
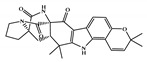
|
prenylated indole alkaloids | marine derived fungus |
0.40 | −8.4 6/−7.4 7 |
| 26845562 1 |
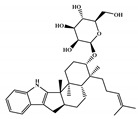
|
indoloditerpenes | marine derived fungus |
0.41 | −8.2 6/−6.9 7 |
| 19384759 1 |
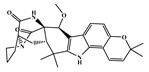
|
prenylated indole alkaloids | marine derived fungus |
0.39 | −8.1 6/−7.3 7 |
| 22435737 1 |
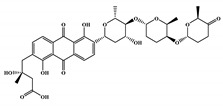
|
anthraquinone | marine derived bacteria | 0.41 | −8.0 6/−7.0 7 |
| 30380253 1 |
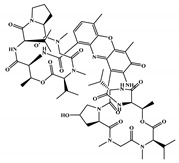
|
phenoxazinone | marine derived bacteria | 0.59 | −8.0 6/−8.5 7 |
| 10714788 1 |
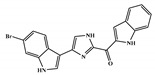
|
bromo deoxytopsentin |
sponge | 0.38 | −7.6 6/−8.3 7 |
| 10720065 1 |
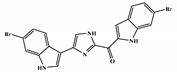
|
dibromodeoxytopsentin | sponge | 0.38 | −7.6 6/−8.5 7 |
| PTM346F6F45 2 |
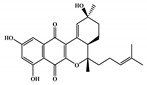
|
marinone | marine derived bacteria | 0.30 | −7.0 6/−5.5 7 |
| nelarabine (Arranon®) 3 |
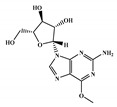
|
purine | sponge | 0.31 | −5.4 6/−5.5 7 |
| fludarabine phosphate (Fludara®) 3 |
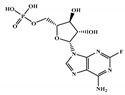
|
purine | sponge | 0.31 | −5.8 6/−6.5 7 |
| nelfinavir 4 |
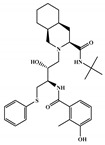
|
octahydro 1H-isoquinoline | --- | --- | −7.4 6/−6.7 7 |
| lopinavir 4 |
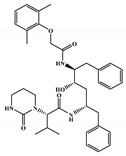
|
2-oxotetrahydro pyrimidine |
--- | --- | −6.5 6/−6.0 7 |
| allicin 5 |
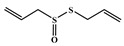
|
diallyl thiosulfinate | --- | --- | −3.3 6/−2.9 7 |
1 Reaxys ID from the fifteen selected MNPs. 2 In-house MNPs library. 3 MNPs clinical pipeline library. 4 Positive controls. 5 Negative control. 6 Mpro enzyme: center X: −36.149 Y: −3.796 Z: 45.045. 7 Mpro enzyme: center X: −12.806 Y: 18.646 Z: 65.607.
