Abstract
Reasons for performing study:
Our long-term aim is to develop a gene therapy approach for the prevention of laminitis in the contralateral foot of horses with major musculoskeletal injuries and non-weightbearing lameness.
Objectives:
The goal of this study was to develop a practical method to efficiently deliver therapeutic proteins deep within the equine foot.
Study design:
Randomised in vivo experiment.
Methods:
We used recombinant adeno-associated viral vectors (rAAVs) to deliver marker genes using regional limb perfusion through the palmar digital artery of the horse.
Results:
Vector serotypes rAAV2/1, 2/8 and 2/9 all successfully transduced equine foot tissues and displayed similar levels and patterns of transduction. The regional distribution of transduction within the foot decreased with decreasing vector dose. The highest transduction values were seen in the sole and coronary regions and the lowest transduction values were detected in the dorsal hoof-wall region. The use of a surfactant-enriched vector diluent increased regional distribution of the vector and improved the transduction in the hoof-wall region. The hoof-wall region of the foot, which exhibited the lowest levels of transduction using saline as the vector diluent, displayed a dramatic increase in transduction when surfactant was included in the vector diluent (9- to 81-fold increase). In transduced tissues, no significant difference was observed between promoters (chicken β-actin vs. cytomegalovirus) for gene expression. All horses tested for vector-neutralising antibodies were positive for serotype-specific neutralising antibodies to rAAV2/5.
Conclusions:
The current experiments demonstrate that transgenes can be successfully delivered to the equine distal extremity using rAAV vectors and that serotypes 2/8, 2/9 and 2/1 can successfully transduce tissues of the equine foot. When the vector was diluted with surfactant-containing saline, the level of transduction increased dramatically. The increased level of transduction due to the addition of surfactant also improved the distribution pattern of transduction.
Keywords: horse, gene therapy, recombinant adeno-associated viral vectors, neutralising antibodies, regional limb perfusion, viral transduction
Introduction
Laminitis affects all breeds of horses and results in intense suffering for those afflicted with its most severe forms. The encased location of the epidermal and dermal lamellae make laminitis challenging to treat. The same traits that make laminitis difficult to treat also make laminitis a particularly good candidate for a gene therapy approach. Gene therapy uses viruses as vehicles to carry ‘good’ genes into a patient’s cells to fight or prevent disease. Viruses efficiently infect difficult-to-access tissues. To treat laminitis, gene therapy would use a virus to insert a therapeutic gene into the cells of the foot. These ‘infected’ cells will then produce a therapeutic protein within the foot to block the enzymatic degradation of lamellar tissues associated with laminitis.
In the current study, we chose to use recombinant adeno-associated viral vectors (rAAVs) because AAVs are very proficient at infecting cells and tissues and possess specific cell and tissue tropisms: the ability to transduce different types of cells or tissues with a range of efficiencies [1,2]. This characteristic can be exploited to target specific tissues in vivo. Attachment to and entry into susceptible cells is mediated by the protein envelope on the surface of a virus. Some specific tropisms include: AAV1 (retina, pancreas), AAV2 (liver, kidney), AAV5 (lung, retina), AAV6 (heart, lung), AAV7 (retina), AAV8 (liver, retina, pancreas, heart) and AAV9 (liver, heart, brain, lungs, pancreas, kidney).
Adeno-associated viruses are safe to use and are currently being used in human clinical trials. Adeno-associated virus is an infectious human virus with no known disease association. It is predominantly a nonintegrating vector; it rarely inserts its viral DNA into the chromosomal DNA of the host. Because of this it is associated with a greatly reduced risk of causing insertional mutagenesis or disrupting the patient’s DNA. It is known to be less immunogenic than are other commonly used adenoviral vectors and can provide long-term expression in a variety of target tissues. To prepare AAV for gene therapy, we remove the genes for viral replication (‘modified virus’), insert the genes we want to transfer (therapeutic or marker gene) and the virus will then induce the production of the desired protein in the virus-infected cells and tissues, but the virus cannot proliferate. By replacing nonessential viral genes with desired beneficial genes, recombinant viruses can be used to deliver therapeutic genes and proteins to target tissues proficiently.
Our specific long-term goal is to develop a gene therapy approach for the prevention of laminitis in the contralateral foot of horses with major musculoskeletal injuries. We hypothesise that gene therapy can be used in vivo to protectively modify the internal tissue environment of the distal extremity without disrupting normal hoof architecture and that a rAAV can be used to transfer a therapeutic gene to equine foot tissue using local vascular perfusion. The current study was designed to 1) determine the optimal rAAV vector serotype and dose for effective transduction of tissue within the equine distal extremity, and 2) determine the biodistribution pattern of molecular markers following regional intra-arterial perfusion with a rAAV vector into the equine foot. Our approach will add a novel research and potentially therapeutic tool to the currently available options for investigating, treating and preventing laminitis.
Materials and methods
Experimental design
Seven (of 13 candidate animals) adult mixed-breed horses were used. Horses were selected on the basis of physical examination and digital radiographs, and were free from sepsis, chronic drug therapy and laminitis. Ages ranged from 12 to 26 (mean 21) years with body weights between 437 and 683 kg (mean 544 kg). Animals were kept at pasture in groups. Horses were randomly assigned to serotype/dose/surfactant treatment groups. Seven horses received rAAV vector diluted in saline or surfactant in both front feet and samples were collected between 7 and 21 days post vector injection.
Vector production
Six different rAAV vector configurations were used (Table 1) including serotypes 2/1, 2/5, 2/8 and 2/9 with chicken β-actin (CB) and cytomegalovirus (CMV) promoters (to promote/enhance expression of the transgene), and a β-galactosidase (β-gal) transgene with a nuclear localisation signal (nLacZ) or an enhanced green fluorescent protein (eGFP) transgene. Our vector configurations were based on the AAV2 viral genome packaged in different serologically distinct capsids, which altered the distribution of the vector DNA and host cell range, allowing us to preferentially direct the recombinant virus to different tissue types. AAV2/1, for example, is the AAV2 genome packaged in the AAV1 capsid serotype and AAV2/9 is the AAV2 genome packaged in the AAV9 capsid serotype. Recombinant AAV2-CMV-eGFP, rAAV2-CB-nLacZ and the rAAV2/1, 2/5, 2/8 and 2/9 packaging constructs were generated and vectors were produced by triple transfection and purified as previously described [3–6]. Briefly, a CMV- or CB-driven transgene cassette encoding eGFP or nLacZ, flanked by AAV2 inverted terminal repeats, was co-transfected into cells from the human embryonic kidney (HEK) 293 cell line with a packaging plasmid encoding the rep and cap genes. Adenoviral helper function was provided in trans from a third helper plasmid, pDF6. Recombinant AAV vector purification was performed as previously described [6]. All vector preparations were banded on 3 sequential CsCl density gradients. The bovine growth hormone polyadenylation sequence (bGH) was included in all vectors to allow titre and biodistribution analysis, regardless of the vector configuration used. Vector titres were assessed by TaqMana qPCR with primers and a probe specific for the polyadenylation signal in the vector transgene cassette (bGH forward: GCCAGCCATCTGTTGT; bGH reverse: GGAGTGGCACCTTCCA; bGH Probe: 6FAM (fluorescein-fluorescence donor) - TCC CCC GTG CCT TCC TTG ACC - TAMRA (carboxytetramethylrhodamine quencher)). All rAAV vector configurations used in the current study were previously tested in vitro and ex vivo in equine cells and tissues [1,7].
TABLE 1:
Viral vector injection scenario into the equine distal extremity
| Horse | Foot | Serotype | Promoter | Transgene | Dose | Diluent | Duration (days) |
|---|---|---|---|---|---|---|---|
| A | L | rAAV 2/8 | CB | nLacZ | 1.0 × 1013 | Saline | 7 |
| R | rAAV 2/5 | CB | nLacZ | 1.0 × 1013 | Saline | ||
| B | L | rAAV 2/8* | CMV | eGFP | 1.0 × 1013 | Saline | 7 |
| R | rAAV 2/5 | CMV | eGFP | 1.0 × 1013 | Saline | ||
| C | L | rAAV 2/8 | CB | nLacZ | 6.0 × 1013 | Saline | 7 |
| R | rAAV 2/1 | CB | nLacZ | 6.0 × 1013 | Saline | ||
| D | L | rAAV 2/8* | CMV | eGFP | 3.0 × 1013 | Saline | 21 |
| R | rAAV 2/8* | CMV | eGFP | 3.0 × 1013 | Surfactant | ||
| E | L | rAAV 2/8 | CB | nLacZ | 2.0 × 1013 | Saline | 7 |
| R | rAAV 2/8 | CB | nLacZ | 6.0 × 1012 | Saline | ||
| F | L | rAAV 2/9 | CB | nLacZ | 1.0 × 1013 | Saline | 7 |
| R | rAAV 2/9 | CB | nLacZ | 1.0 × 1013 | Surfactant | ||
| G | L | rAAV 2/9 | CB | nLacZ | 1.0 × 1013 | Saline | 14 |
| R | rAAV 2/9 | CB | nLacZ | 1.0 × 1013 | Surfactant |
L, left; R, right.
This vector had been previously thawed and refrozen, which may have compromised its potency. Dose = viral copies (VC) injected.
Animal procedures
Horses were placed in lateral recumbency under general anaesthesia and both front fetlocks and pasterns were clipped and scrubbed with antiseptic solutions. A 2 cm incision was made directly over the neurovascular bundle coursing over the abaxial, uppermost proximal sesamoid bone. The palmar digital artery was bluntly dissected from the bundle and a 14 gauge over-the-needle intravenous catheter (Arrow catheter)b was inserted. The incision was closed over the catheter to allow more distal positioning of a pneumatic tourniquet. The top of the tourniquet was positioned in the mid-metacarpal region and extended distally over the metacarpophalangeal joint and was inflated to 400 mmHg (Fig 1a).
Fig 1:
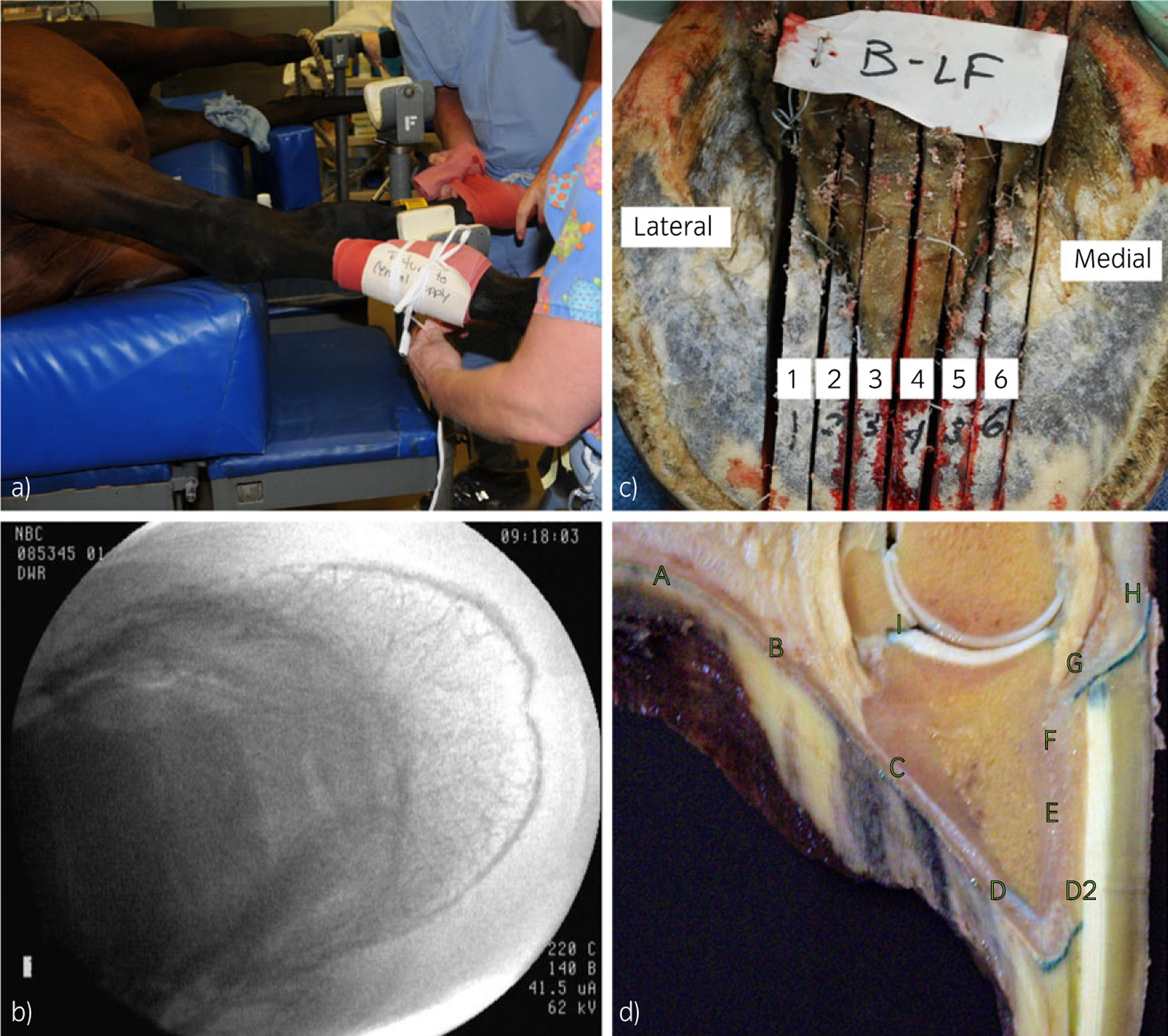
Vector delivery and tissue recovery. a) Tourniquet placement to occlude blood flow to the distal extremity. b) Fluoroscopic image showing complete vascular filling with positive contrast. c) Left-front foot sectioned sagittally from lateral to medial for tissue recovery. d) Regional tissue harvesting from site A to site I, taken from an in situ X-gal-stained hoof slice.
Fluoroscopy, using the iodinated contrast agent/fluorophore iohexol (Omnipaque; 240 mg/ml)c was used to check that the position of the catheter tip was at the level of the distal sesamoid (navicular) bone, and to assess vascular filling by allowing visualisation of the injected fluid filling the vasculature of each foot (Fig 1b). At a 25 ml volume, each foot was completely opacified. After complete filling was confirmed, the tourniquet pressure was released briefly to allow pressure to return to normal within the vasculature of the foot. The tourniquet was subsequently re-inflated and 25 ml of isotonic saline containing rAAV with or without the addition of 0.01% Pluronic F68 surfactant (Sigma-Aldrich)d was injected into the foot. All 7 horses received vector in both front feet (Table 1). Marker genes were packaged in 6 different configurations and 3 dose levels (low = 6.0 × 1012, medium = (1.0–3.0) × 1013 and high = 6.0 × 1013 viral copies (VC)). The dose is the number of copies of viral genome delivered (VC injected). The tourniquet remained inflated for 30 min after which the tourniquet and catheter were removed, limbs were bandaged routinely, an anti-inflammatory (phenylbutazone, 4.4 mg/kg bwt, i.v.) was administered and the horses were allowed to recover from general anaesthesia. All horses appeared normal post operatively with no signs of lameness and were housed in pasture conditions after recovery.
Tissue retrieval
Each horse had 7–21 days of normal activity and was then subjected to euthanasia following the 2000 AVMA Guidelines (median experiment duration = 7 days, Table 1). Both front and one hind foot, axillary lymph nodes, spleen, lung, kidney, brain, bone marrow and liver tissues were harvested at necropsy. Tissue retrieval began within a few minutes of euthanasia. The feet were disarticulated at the metacarpophalangeal joint and each foot was sectioned sagittally into 8 slices using a band saw (Fig 1c). Tissue samples were collected from 10 distinct regions of each slice (labelled A–I, as in Fig 1d) using a No. 22 stainless steel surgical blade from the proximal (F), middle (E) and distal areas (D2) of the dorsal hoof-wall region, from the palmar/plantar (A), axial (B and C) and dorsal (D) sole regions, and from the proximal (H) and distal (G) areas of the coronary band. Synovial tissue was collected from the distal interphalangeal joint (I) using iris dissection scissors and consisted of synovial, adipose and fibrous capsular tissue. Biodistribution of the injected rAAV vectors was assessed using 1) real-time qPCR and 2) histological assessment of whole and sectioned hoof tissue.
Histological analysis of β-galactosidase transgene expression
β-galactosidase expression was analysed in both cryopreserved tissue sections collected from each hoof region, and in intact, whole hoof slices. Regional tissue samples were placed in histology freezing boats, covered with OCT freezing solutione and placed in liquid nitrogen-cooled isopentane for approximately 30–45 s. The frozen tissue blocks were then placed on dry ice. When all samples had been collected, the frozen samples were placed at −80°C until further processing. Tissue slices cut at 6 µm were placed on slides, subjected to a quick fixation with paraformaldehyde and processed routinely for β-gal expression [8]. Whole, intact hoof slices were analysed in situ for β-gal expression (Fig 1d). Intact hoof slices were collected, rinsed and stained at room temperature (21°C) for 24 h for β-gal expression. In cryosectioned and whole slice staining protocols, pH was maintained at or slightly above 7.0 to suppress endogenous β-gal enzyme activities. After staining for β-gal expression, the hoof slices were fixed in 10% neutral buffered formalin for 48 h. β-galactosidase-stained, intact hoof slices were imaged grossly with a digital camera and subsequently tissue from each region was removed and processed routinely for paraffin sectioning [8].
Synoviocytes may produce significant endogenous levels of lysosomal galactosidase, an enzyme that may react with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) during the β-gal staining procedure and produce false-positive signals [9]. We used a nuclear-targeted nLacZ gene to more easily identify β-gal staining that was due to vector injection, as opposed to endogenous LacZ-positive signals from lysosomal galactosidase in synoviocytes (Fig 2). Nontransduced tissues were included during the staining procedures as negative controls. Sections were evaluated for cell/tissue-specific transgene expression by a board-certified pathologist (J.B.E.).
Fig 2:
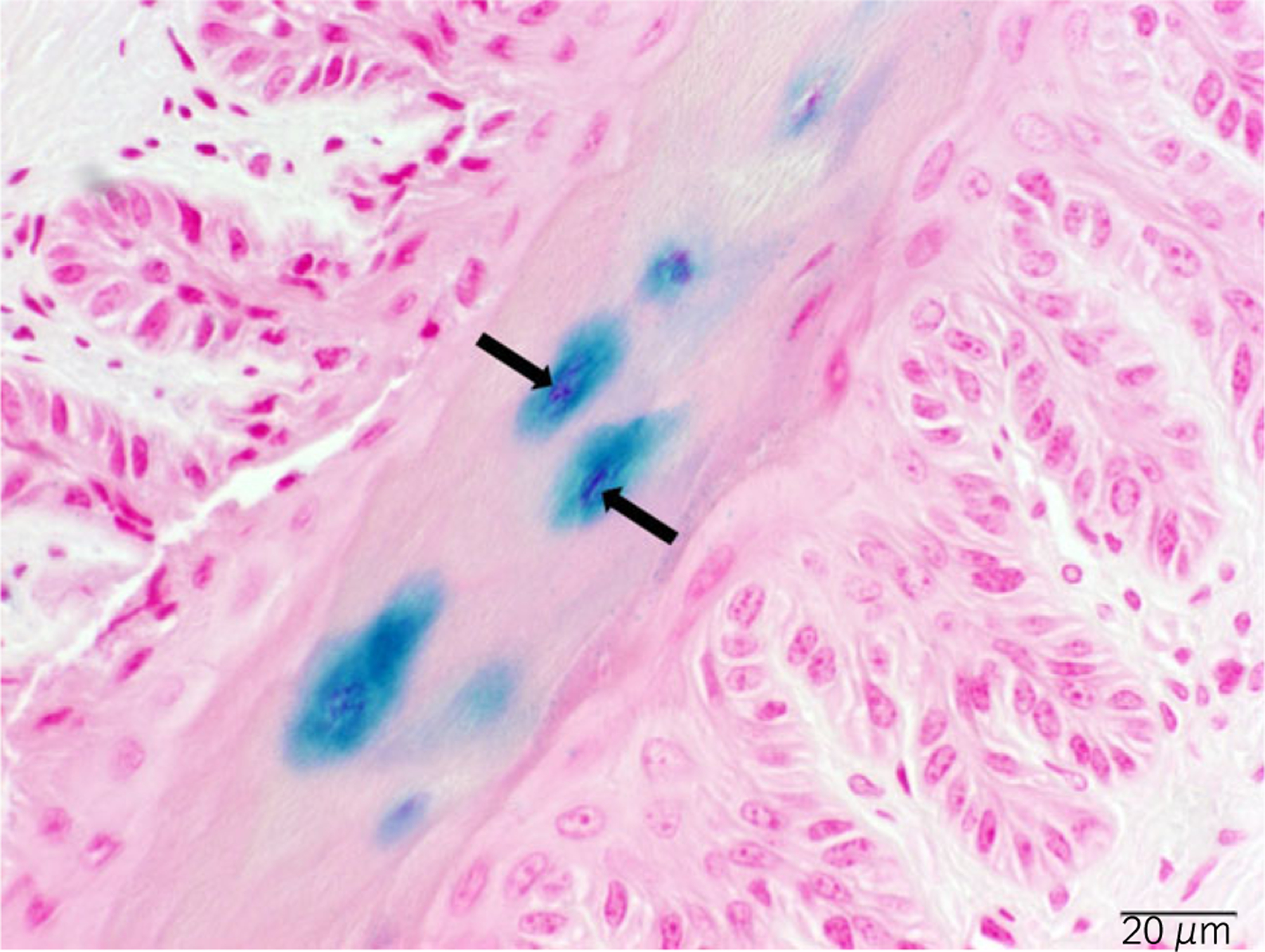
Nuclear-targeted nLacZ. Primary epidermal lamellae, considered to be anuclear in some regions, display robust nuclear β-galactosidase (β-gal) staining. This β-gal staining was due to vector injection because of the obvious dark staining originating in the nucleus. The arrows indicate positive nuclear β-gal (blue) staining.
Regional quantification of vector copy number
Frozen tissue specimens were used for the isolation of DNA, which was subsequently used for the determination of the ratio of virus particles inside cells to the total number of cells in the sample. More specifically, the ratio of copies of the vector genome incorporated into cells in specific regions of the foot to copies of endogenous genomic DNA (cell number). Vector biodistribution analysis was performed by qPCR as described previously [10]. Briefly, total cellular DNA was extracted from freezer millpulverised hoof tissue (SPEX)f using a QIAamp DNA Mini Kit (Qiagen)g. Detection and quantification of vector genomes in extracted DNA were performed by qPCR using primer and probe sets targeted to the polyadenylation signal in the vector transgene cassette (bGH forward: GCCAGCCATCTGTTGT; bGH reverse: GGAGTGGCACCTTCCA; bGH Probe: 6FAM - TCC CCC GTG CCT TCC TTG ACC - TAMRA). The qPCR conditions were set at 100 ng total cellular DNA as template, 300 nmol/l primers and 200 nmol/l probes each. Cycles were for 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C. Genome copies (GC)/100 ng DNA was determined from the mean value of 3 runs. A value of 6.64 pg DNA per diploid cell was used to approximate endogenous genome copy number.
Neutralising antibody analysis
Neutralising antibody analysis was performed (Table 2) on serum samples collected from 13 candidate horses pre-vector injection and from 7 experimental horses post vector injection by the Immunology Core at the University of Pennsylvania as previously described [11]. Briefly, serum samples were heat inactivated at 56°C for 35 min. All vectors, including serotypes 2/1, 2/2, 2/5, 2/6, 2/8 and 2/9 were diluted (109 GC/well) in serum-free Dulbecco’s modified Eagle’s medium (DMEM) and incubated with 2-fold serial dilutions (initial dilution, 1:20) of heat-inactivated serum samples in DMEM for 1 h at 37°C. Subsequently, the serum–vector mixture was added to 96-well plates seeded with 1 × 105 hepatocyte derived cellular carcinoma (Huh7) cells/well that had been infected 2 h earlier with wild-type HAdV5 (50 VC/cell). After 1 h, each well was supplemented with an equal volume of 20% fetal bovine serum (FBS)/DMEM and incubated for 18–22 h at 37°C and 5% CO2. The cells were then washed twice in PBS and lysed, and the lysate was measured in a microplate reader. The neutralising antibody (NAb) titre was reported as the highest serum dilution that inhibited transduction for each vector by >50% compared with a mouse serum control.
TABLE 2:
Equine plasma samples tested for pre-existing neutralising antibodies to adeno-associated viral vectors
| Vector serotype | Plasma samples (candidate animals) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| rAAV 2/9 | <5 | <5 | <5 | ||||||||||
| rAAV 2/8 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| rAAV 2/5 | 10 | 10 | 5 | 10 | 10 | 40 | 80 | 40 | 20 | 20 | 10 | 40 | 20 |
| rAAV 2/6 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | 5 | <5 | <5 |
| rAAV 2/2 | <5 | <5 | <5 | <5 | <5 | <5 | 5 | <5 | <5 | <5 | 5 | <5 | <5 |
| rAAV 2/1 | <5 | ||||||||||||
Values for neutralising antibodies (NAb) are defined as the highest serum dilution that inhibited vector transduction by >50%. Equine plasma from only one horse was tested for NAb to serotype AAV 2/1.
Data analysis
Statistical analysis was conducted with JMPh. A Shapiro–Wilk test was used to determine normality. Experimental data were not normally distributed and were subsequently transformed (1/-log10) and analysed with two-factor ANOVA without replication. Individual treatments were further analysed by paired Student’s t test, two-tailed, unequal distribution of variance assumed. Test results were considered significant for P values ≤0.05.
Results
To compare the results of experiments where multiple vector dosages were used, we normalised the results to the dose of vector injected. For example, if we injected 1.0 × 1012 copies of viral genome into an animal and we detected 2 viral copies (VC) in 10 cells (GC), this would be expressed as 2.0 × 10−13 VC/GC/VC injected. This reflects the efficiency of the vector to transduce the tissue in the region of interest. The patterns of normalised efficiency mirror the patterns of absolute transduction (data not shown). Values in tissues from noninjected hind feet or in any organs or any other tissues tested were always below the level of detection in our real-time qPCR assay. Copy-number data include all vectors used, with the exception of serotype 2/5 vectors. Histology results include data from nLacZ-containing vectors only. Due to the presence of endogenous NAb for serotype 2/5, no copy number or histology data are reported for experiments that used a 2/5 vector.
Serotype effects
Increased tissue transduction was obtained with vector serotype 2/8 compared with serotype 2/9 (Fig 3a) and with serotype 2/1 (Fig 3b). Transduction values within a foot were always greatest in a central section (slices 3 and 4, Fig 1c) compared with lateral and medial sections of the foot. Transduction with serotype 2/5 was not detected in any tissue samples tested (presumably due to neutralising antibodies to serotype 2/5).
Fig 3:
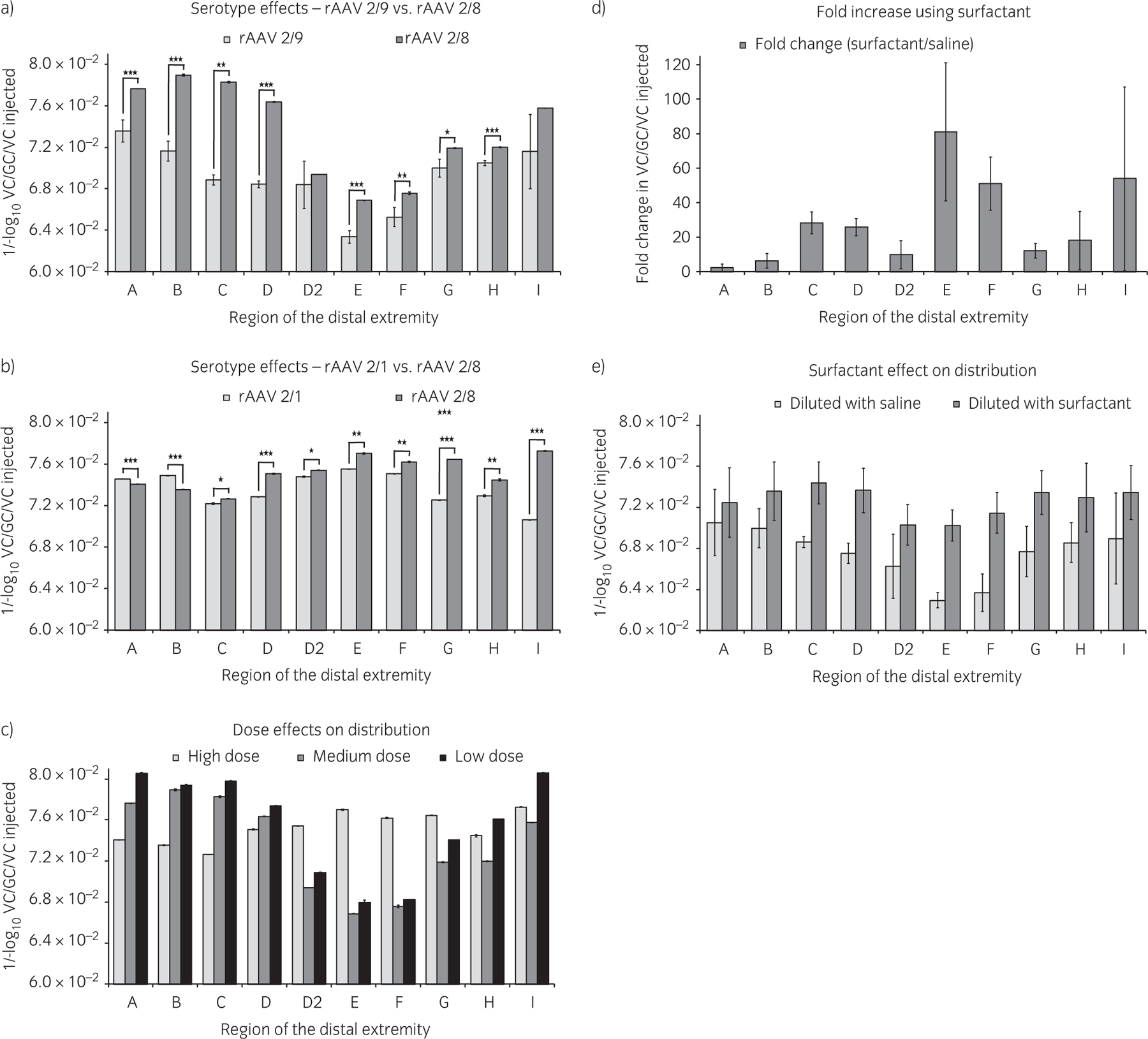
Effects on vector transduction. a) Serotype 2/8 displayed increased tissue transduction compared with serotype 2/9. b) Serotype 2/8 also displayed increased transduction compared with 2/1. c) Dose influence on regional distribution of transduction was only affected at the highest dose (reported for serotype 2/8). d) Surfactant increased transduction in difficult-to-transduce regions (average change with serotypes 2/8 and 2/9). e) Surfactant improved regional transduction patterns compared with saline (average change with serotypes 2/8 and 2/9). Values for serotype 2/8 from graph (a) (medium dose) and graph (b) (high dose) are from separate experiments. Error bars represent s.e. *P<0.10, **P<0.05, ***P<0.01. rAAV = recombinant adeno-associated viral vector.
Dose effects
When comparing vector dosage, the dose of vector injected had a significant effect on the distribution of vector transduction within various hoof tissues (all P values were <0.05, with the exception of medium to low dose at regions A (P = 0.07), E (P = 0.1) and F (P = 0.09), Fig 3c). Low (6.0 × 1012 VC) and medium doses ((1.0–3.0) x 1013 VC) of vector displayed very similar transduction patterns and reflected the circulation pattern within the foot from the point of vector injection at the level of the distal sesamoid bone. The dorsal hoof wall region displayed the lowest transduction efficiency. The use of high doses of vector (6.0 × 1013 VC) produced a different pattern of regional transduction compared with the use of medium and low doses, that is, a more even distribution throughout the foot. In the medium dose groups, no significant difference in regional or level of transduction was detected between doses of 1.0 × 1013 VC, 2.0 × 1013 VC or 3.0 × 1013 VC. However, the lot of vector used for the 3.0 × 1013 VC experiments (serotype 2/8) may have been compromised due to previous sub-optimal handling conditions.
Region-specific transduction
The stratum medium of the sole as well as the coronary lamellar epidermis (corium) were the most heavily transduced tissues within the foot (Fig 4). Epithelial cells were the predominant cell type transduced; cells of the secondary epidermal lamina and hoof tubules were heavily transduced (Fig 4). The images in Figure 4 demonstrate β-gal-stained cells in the suprabasal tubule and intertubular horn (rather than the basal cell layer; basal cells become suprabasal and intertubular horn cells as the hoof grows). Histological analysis of intact foot slices revealed the distal migration of transduced tissue/protein from the coronary band, down the hoof wall (Fig 4d). Nuclear β-gal staining was not detected in tissues from noninjected hind feet or in any organs or any other tissues tested. Because the pH of our X-gal staining solution was maintained at or slightly above pH 7.0, most of the potential endogenous β-gal enzyme activity was suppressed. However, mild cytoplasmic β-gal staining was noted in certain tissues, most notably kidney, brain and bone marrow.
Fig 4:
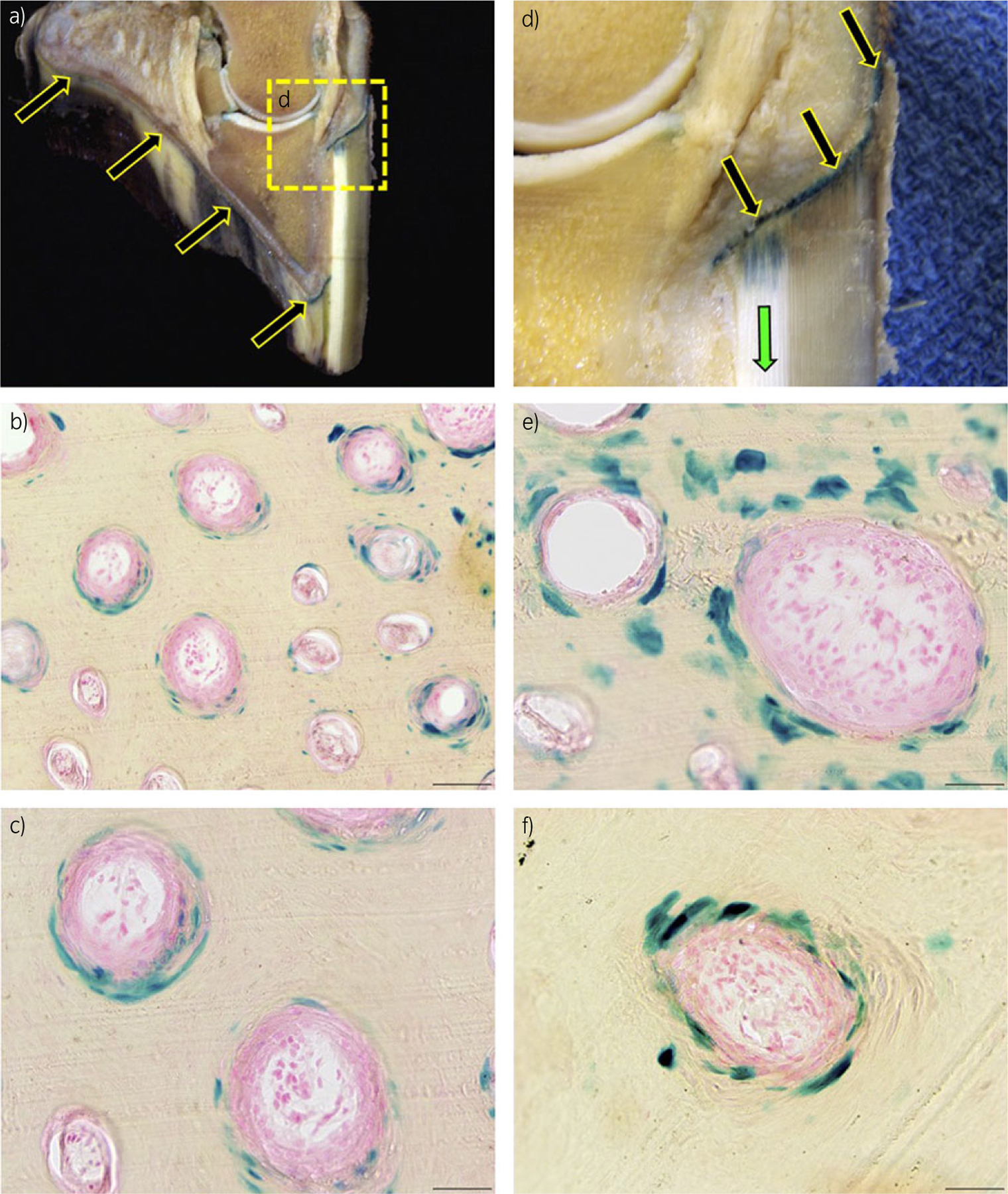
β-galactosidase (β-gal) staining, 7 days post injection. a) Sagittal section of an equine digit. β-galactosidase protein is expressed in the coronary and sole epidermal lamellae and stratum medium (arrows). Images (b), (c), (e) and (f) are representative frozen histological cross-sections of the hoof stratum medium, which display blue, nuclear β-gal staining in cells of the hoof-wall tubules. b) 9200, scale bar = 100 µm. c), e) and f) 9400, scale bar = 50 µm. d) Higher magnification of the coronary epidermis expressing β-gal.
Promoter effects
No significant differences were detected in gene expression between the CB promoter (which was used for most of the experiments) and the CMV promoter. In contrast to the potential effects on gene expression, values for tissue transduction/copy number are not typically influenced by promoter type.
Surfactant effects
When the vector was diluted in saline that included 0.01% surfactant, the level of transduction increased dramatically in all hoof regions compared with vector diluted in saline alone (Fig 3d). The use of surfactant increased the level of transduction to a greater degree in the regions of the foot that were the most difficult to transduce (hoof wall) and showed the least benefit in easily-transduced regions (sole and coronary). This included an 81-fold increase in transduction in the centre of the hoof wall (region E) and a 51-fold increase in the proximal hoof-wall region F (Fig 1d). The addition of surfactant to a moderate dose of vector significantly changed the regional distribution of transduction within the distal extremity to a pattern only seen previously with a 6-fold higher dose of vector (Fig 3e).
Pre-existing neutralising antibodies
Subsequent to the first 2 experiments (serotype 2/5 and 2/8 vector injections), but prior to additional vector injections, the first 2 injected horses and 11 additional potential gene therapy candidates (13 horses total) were screened for pre-existing NAbs to serotypes 2/2, 2/5, 2/6 and 2/8. Serum samples were collected before and after vector injection from all injected horses. Every animal that was tested before vector injection was positive (≥5) for NAbs to serotype 2/5, 2 of 13 were positive for NAb to serotype 2/2 and one horse was positive for NAbs to serotype 2/6. In addition to this initial screen, of the 13 screened candidate horses, 3 animals received supplemental screening for serotype 2/9 (all negative, <5) and one animal received supplemental screening for serotype 2/1 (negative, Table 2). Data are not available for post injection NAbs for all animals, but representative values for animals that tested negative pre-injection were between 40 and 320 post injection. Animals that tested positive pre-injection were always ≥640 post injection. Seven of the initial 13-horse pool of candidates were used in these experiments.
Discussion
The current results demonstrate successful in vivo transduction of equine hoof tissues with a rAAV vector. In the current experiments, serotypes 2/1 and 2/9 were marginally less efficient than serotype 2/8 in the equine foot. These differences in transduction averaged only 2% and 6% for serotype 2/8 compared with serotypes 2/1 and 2/9, respectively. In previous in vitro experiments, serotypes 2/8 and 2/9 both demonstrated poor performance in equine diarthrodial joint cells and tissues. In these previous experiments, serotype 2/1 demonstrated more promising levels of transduction [1]. In contrast to these in vitro results, previous in vivo gene therapy results with serotype 2/9 demonstrated superior performance [12].
Delivery of treatments to the equine foot is significantly limited by the innate architecture of the hoof. However, there is abundant communicating vascular plexus throughout the foot, so pressurised fluid placed in the palmar digital artery is efficiently distributed. The profuse vascular plexus within the equine foot probably offered an advantage to the vector serotype best able to cross blood vessel barriers, giving an advantage to serotype 2/8 [6,13]. However, with n = 1 for serotype 2/1, interpretation of results for this serotype are reserved.
The notable difference in regional transduction of the foot, between the sole and coronary regions and the hoof-wall region was probably influenced by the circulation patterns within the foot. The sole receives a rich blood supply from the circumflex artery of the sole as well as the terminal arch blood supply [14]. This high level of blood supply probably contributed to the increased level of dose-independent vector transduction observed in the sole papilla region of the foot (Fig 1d, regions A–C). The coronary band receives blood supply from the terminal arch blood supply as well as the coronary circumflex artery. This favourable blood supply is reflected as increased transduction in the coronary region (Fig 1d, regions G–H), compared with the hoof-wall lamellar layer. The low level of blood supply from the lamellar arteries in the secondary dermal lamina presents a difficult transduction scenario for the lamellar layer inside the hoof wall (Fig 1d, regions E–F), particularly at low vector doses.
As the vector dose increased, the apparent preferential transduction of the sole and coronary regions was significantly reduced. The high-dose and surfactant-supplemented injections appear to have circumvented this blood-supply disadvantage in the hoof-wall region. The more easily transduced sole and coronary regions may have become saturated with vector when we used surfactant and at high vector doses. These conditions produced the appearance of a comparatively even distribution of vector throughout the foot. All of our vector injections were performed with the animals in lateral recumbency. The vector distribution patterns may be different in a standing animal.
In situ β-gal staining of the intact hoof slice provided a valuable context in which to observe the distal migration of transduced cells/tissues/proteins from the coronary band toward the toe, in the direction of hoof growth only 1 week after vector injection. Previous in vivo results showed that gene expression can increase significantly between Day 7 and Day 28 [12]. Conversely, multiple rAAV vector studies identify a drop in transgene expression over time due to pre-existing immune responses to vector capsid proteins and acquired responses against vector and transgene products, as well as high cell turnover [15,16]. The histological observations seen here could have been influenced by any of these variables. In the current study, gene expression was no different between 7- and 14-day incubation times (time from injection to collection), with or without surfactant, in horses F and G. Due to variations in vector dose and diluent, other comparisons of incubation time were not valid.
The CB promoter historically produces higher levels of expression than the CMV promoter, which is prone to silencing over time in some tissues [17–19]. In the current experiments using the CB promoter, gene expression in vivo occurred at the earliest time point checked, 1 week, and was no different at 2 weeks, the latest time point for the CB promoter. The CMV promoter also produced strong gene expression at the 7- and 21-day incubation times. Incubation times from 7 to 21 days were unlikely to expose potential long-term gene silencing in these experiments. However, in previous experiments in our lab, gene expression with a CB promoter continued for a minimum of 1 year [12].
Previously, the inclusion of surfactant as a component of the vector diluent increased in vivo gene expression in rodent stifle joints, as measured with in vivo bioluminescence imaging [12,20]. In the current experiments in the horse, when vector was diluted with surfactant-containing saline for delivery to the equine foot, the level of transduction increased dramatically. The ability of surfactant to increase transduction at a decreased dose could allow the use of a lower in vivo viral titre, which would minimise the risk of NAb formation and potentiate repeat dosing.
Serotype-specific NAbs can preclude successful transduction [21,22]. After our first 2 failed attempts at transduction of the equine distal extremity with serotype 2/5, these animals and each subsequent candidate animal was tested for NAbs to serotypes 2/2, 2/5, 2/6 and 2/8. To our surprise, 100% of the horses tested were positive for NAbs to serotype 2/5. Among this test group, serotype 2/8 was the only serotype tested that did not reveal a single NAb-positive animal [23–25]. In addition, 3 animals were also tested for serotype 2/9 and one was tested for serotype 2/1. None of these tests revealed NAb-positive animals.
Conclusions
The current experiments demonstrate that transgenes can be successfully delivered to the equine distal extremity using rAAV vectors and that serotypes 2/8, 2/9 and 2/1 can successfully transduce tissues of the equine foot. When the vector was diluted with surfactant-containing saline, the level of transduction increased dramatically. The increased level of transduction due to the addition of surfactant also improved the distribution pattern of transduction.
To move toward our long-term goal of providing a practical clinical therapy for the treatment/prevention of laminitis, a next logical step is to perfect rAAV vector injection in a standing horse with a procedure more similar to a standard regional limb perfusion. Our laboratory has cloned the equine tissue inhibitor of metalloproteinase-3 gene. The product of this gene, TIMP-3, is known to specifically block several of the most destructive enzymes that destroy the hoof–coffin bone connection. While TIMP-3 is a logical choice to address the devastating degradation associated with laminitis, it is possible that TIMP-3 may not be the ultimate gene therapy ‘target’. The extensive work being done now by other researchers exploring the mechanisms of the disease may identify more beneficial genes in the future, but this study should provide a strong basis for further investigations using gene therapy in equine laminitis.
Acknowledgements
We gratefully acknowledge Drs Hannah Galantino-Homer, Peter Bell, Luk Vandenberghe and Roberto Calcedo, and Mr Ralph Conti and Mr David Lorom, for their intellectual support and technical assistance.
Sources of funding
Research was supported by the Grayson-Jockey Club Research Foundation, Inc., the United States Equestrian Federation, Equine Health Research Fund, the University of Pennsylvania Vector Core Facility and grants to J.M. Wilson (NIH grant 2-P30-DK047757-16). Recombinant AAV preparations were provided by The University of Pennsylvania Vector Core Facility (Philadelphia, USA).
Authors’ declaration of interests
J.M. Wilson is an advisor to REGENXBIO, Dimension Therapeutics, Solid Gene Therapy, and Alexion, and is a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO and Dimension Therapeutics; in addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. All other authors declare that there are no conflicts of interest.
Ethical animal research
All protocols were approved by the Institutional Animal Care and Use Committee of The University of Pennsylvania.
Manufacturers’ addresses
Applied Biosystems, Foster City, California, USA.
Arrow International, Reading, Pennsylvania, USA.
GE Healthcare, Princeton, New Jersey, USA.
Sigma-Aldrich, St Louis, Missouri, USA.
Tissue-Tek, Sakura Finetek, Torrance, California, USA.
SPEX, Metuchen, New Jersey, USA.
Qiagen, Valencia, California, USA.
SAS, Cary, North Carolina, USA.
References
- 1.Mason JB, Vandenberghe LH, Xiao R, Wilson JM and Richardson DW (2012) Influence of serotype, cell type, tissue composition, and time after inoculation on gene expression in recombinant adeno-associated viral vector-transduced equine joint tissues. Am. J. Vet. Res 73, 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelfelder S and Trepel M (2009) Adeno-associated viral vectors and their redirection to cell-type specific receptors. In: Tissue-Specific Vascular Endothelial Signals and Vector Targeting, Part A, Eds: Pasqualini R and Arap W, Elsevier Academic Press Inc., San Diego: pp 29–60. [DOI] [PubMed] [Google Scholar]
- 3.Xiao X, Li J and Samulski RJ (1998) Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol 72, 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auricchio A, Hildinger M, O’Connor E, Gao GP and Wilson JM (2001) Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther 12, 71–76. [DOI] [PubMed] [Google Scholar]
- 5.Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N and Wilson JM (2001) Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol 75, 6199–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao GP, Alvira MR, Wang LL, Calcedo R, Johnston J and Wilson JM (2002) Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. U.S.A 99, 11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason JB, Vandenberghe LH, Wilson JM and Richardson DW (2009) When selecting an adeno-associated viral vector serotype, cell monolayer transduction efficiency does not accurately predict tissue transduction efficiency in equine synovial tissues. FASEB J 23, 817.7. [Google Scholar]
- 8.Bell P, Limberis M, Gao GP, Wu D, Bove MS, Sanmiguel JC and Wilson JM (2005) An optimized protocol for detection of E. coli β-galactosidase in lung tissue following gene transfer. Histochem. Cell Biol 124, 77–85. [DOI] [PubMed] [Google Scholar]
- 9.Roessler BJ and Davidson BL (1993) Genetic modification of synoviocytes in vivo using recombinant adenoviral vectors. Clin. Res 41, A171. [Google Scholar]
- 10.Bell P, Moscioni AD, McCarter RJ, Wu D, Gao GP, Hoang A, Sanmiguel JC, Sun X, Wivel NA, Raper SE, Furth EE, Batshaw ML and Wilson JM (2006) Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol. Ther 14, 34–44. [DOI] [PubMed] [Google Scholar]
- 11.Calcedo R, Vandenberghe LH, Gao GP, Lin JP and Wilson JM (2009) Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis 199, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason JB, Gurda BL, Engiles JB, Hankenson KD, Wilson JM and Richardson DW (2013) Multiple recombinant adeno-associated viral vector serotypes display persistent in vivo gene expression in vector-transduced rat stifle joints. Hum. Gene Ther. Methods 24, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Zhu T, Qiao CP, Zhou LQ, Wang B, Zhang J, Chen CL, Li J and Xiao X (2005) Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol 23, 321–328. [DOI] [PubMed] [Google Scholar]
- 14.Mishra PC and Leach DH (1983) Extrinsic and intrinsic veins of the equine hoof wall. J. Anat 136, 543–560. [PMC free article] [PubMed] [Google Scholar]
- 15.Bell P, Gao G, Haskins ME, Wang L, Sleeper M, Wang H, Calcedo R, Vandenberghe LH, Chen S-J, Weisse C, Withnall E and Wilson JM (2011) Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs. Hum. Gene Ther 22, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Wang H, Bell P, McMenamin D and Wilson JM (2012) Hepatic gene transfer in neonatal mice by adeno-associated virus serotype 8 vector. Hum. Gene Ther 23, 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raisler BJ, Berns KI, Grant MB, Beliaev D and Hauswirth WW (2002) Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1–3 of angiostatin reduce retinal neovascularization. Proc. Natl Acad. Sci. U.S.A 99, 8909–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, Wolfe R, Visel M, Stone D, Libby RT, DiLoreto D Jr, Schaffer D, Flannery J, Williams DR and Merigan WH (2011) Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci 52, 2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, Ehlers MD, Zylka MJ, McCown TJ and Samulski RJ (2011) Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther 22, 1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang FL, Jia SQ, Zheng SP and Ding W (2011) Celastrol enhances AAV1-mediated gene expression in mice adipose tissues. Gene Ther 18, 128–134. [DOI] [PubMed] [Google Scholar]
- 21.Davidoff AM, Gray JT, Ng CYC, Zhang YB, Zhou JF, Spence Y, Bakar Y and Nathwani AC (2005) Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther 11, 875–888. [DOI] [PubMed] [Google Scholar]
- 22.Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E, Bercury SD, Pande NN, Souza DW, Bree MP, Lukason MJ, Marshall J, Cheng SH and Scheule RK (2010) Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol. Ther 18, 1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcedo R, Franco J, Qin Q, Richardson DW, Mason JB, Boyd S and Wilson JM (2011) Pre-existing neutralizing antibodies to AAV capsids in large animals other than monkeys may confound in vivo gene therapy studies. Mol. Ther 19, Suppl. 1, S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathwani AC, Gray JT, McIntosh J, Ng CYC, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EGD and Davidoff AM (2007) Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood 109, 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, Sanmiguel J, Desai RA, Chen CS, Johnston J, Grant RL, Gao G and Wilson JM (2006) Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med 12, 967–971. [DOI] [PubMed] [Google Scholar]


