Figure 1.
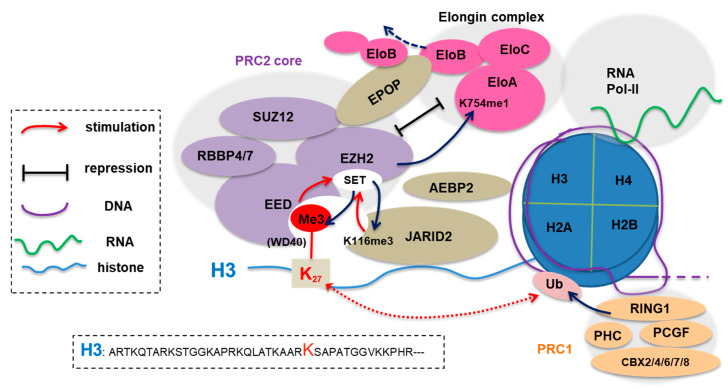
Enhancer of zeste homolog 2’s (EZH2) canonical function as enzymatic subunit of Polycomb repressive complex 2 (PRC2), which additionally consists of embryonic ectoderm development (EED), suppressor of zeste 12 homolog (SUZ12), and retinoblastoma-binding protein 7/4 (RBBP7/4). EZH2:PRC2 silences transcription at least partly through trimethylation of histone H3 lysine 27 (H3K27me3). PRC1, composed of the PCGF (such as BMI1), RING1, CBX (one of CBX2/4/6/7/8) and PHC subunits, both induces histone H2A lysine-119 mono-ubiquitination (H2AK119ub1) and mediates chromatin compaction via a phase separation-related mechanism [25,26]. Besides inducing H3K27me3, EZH2:PRC2 also methylates its own subunit/cofactors such as EZH2 and JARID2 for PRC2 activation. In particular, both H3K27me3 and JARID2-K116me3 are “recognized” by the WD40 repeat module of EED, inducing allosteric activation and possibly on-chromatin spreading of PRC2. Additionally, via the EPOP-mediated sequestration of EloB/C and PRC2:EZH2-mediated methylation of elongin-A (EloA), PRC2 also suppresses assembly and/or activity of the Elongin complex, thus contributing to suppression of target gene expression.