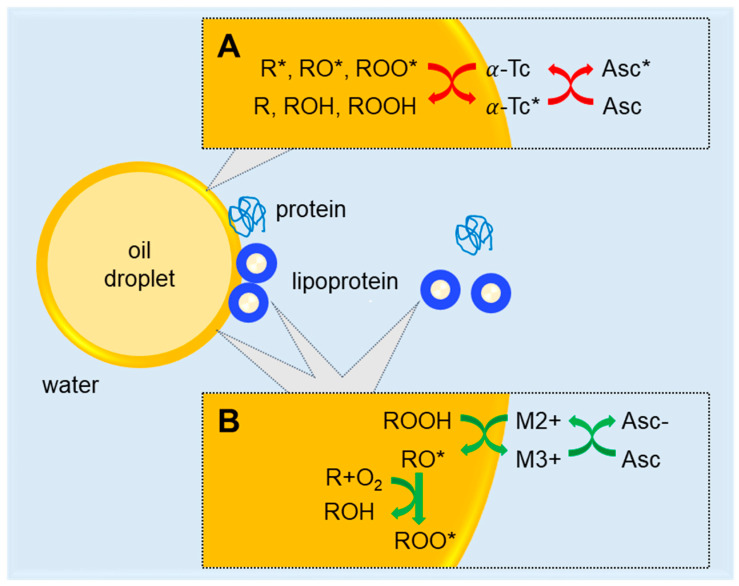
Figure 4.
Overview of the mechanisms of lipid and protein oxidation present in oil-in-water emulsions. Oil droplets are shown in yellow; lipoprotein particles are presented with a yellow lipid core and a blue protein shell. For simplicity, these particles are not shown in their aggregated form in granules. Anti/pro-oxidant mechanisms are depicted at the interfacial droplet layer and at the lipoprotein surface. Anti- and pro-oxidant mechanisms are respectively indicated in red and green. (A) Ascorbic acid acts as an antioxidant for lipids by converting tocopherol radicals to tocopherol. (B) Pro-oxidant behavior of ascorbic acid on lipids and proteins, including lipoproteins, at the interface and the water phase. Mechanisms involve metal chelation as well as free radical scavenging. ROOH: lipid hydroperoxide; R: unsaturated lipids; R*: alkyl radical; RO*: alkoxyl radical; ROO*: peroxyl radical; ROH: hydroxy lipids; Asc: ascorbic acid; Asc−: dehydroascorbic acid; Asc*: ascorbyl radical; Tc: tocopherol; Tc*: tocopherol radical. M2+: Transition metal ions (Fe or Cu).