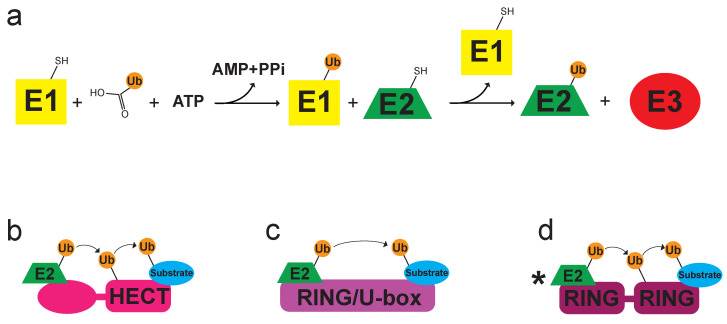
Figure 1.
(a) Schematic representation of the ubiquitination pathway involving E1-E2-E3 enzymes through a concerted, ATP-driven process. There are two major classes of E3 ligases that dictate ubiquitin transfer: (b) homologous to E6AP C-terminal (HECT) domain, where a conserved, catalytic cysteine becomes charged with ubiquitin before the final transfer to substrate; and (c) really interesting new gene (RING)/U-box domain, where the stabilized E2∼Ub conjugate directly transfers ubiquitin from E2 to substrate. There is an additional small class of proteins called (d) RING-in-between-RING (RBR) that behave as a RING/HECT hybrid, where one RING domain recruits an E2 which then transfers ubiquitin to the second RING-like domain.