Fig. 4 |. Effects of nanoparticles on nociceptive, inflammatory and neuropathic nociception.
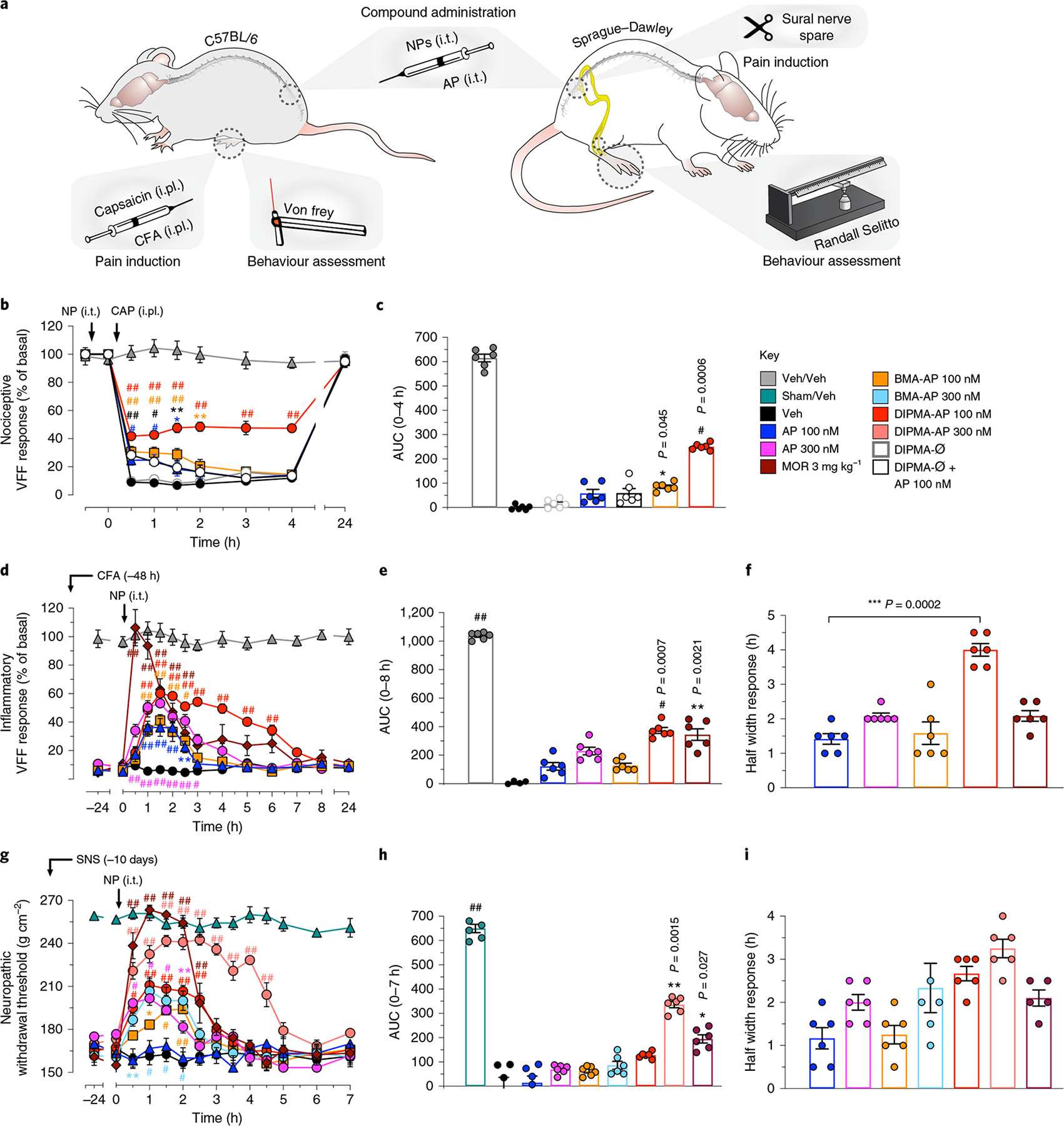
a, Preclinical models of nociceptive, inflammatory and neuropathic pain. In the capsaicin-evoked model of acute nociceptive pain in mice, AP, nanoparticles (NPs) or vehicle (Veh) (5 μl) was injected intrathecally (i.t.) 30 min before intraplantar (i.pl.) injection of capsaicin (CAP) or Veh. Withdrawal responses were measured to stimulation of the plantar surface of the injected hindpaw with VFF. In the complete Freund’s adjuvant (CFA)-evoked model of sustained inflammatory nociceptifon in mice, CFA or Veh was administered by i.pl. injection; after 48 h, AP, NP or Veh was administered by i.t. injection (5 μl). Withdrawal responses were measured to VFF stimulation of the plantar surface of the injected hindpaw. In the sural nerve spared (SNS) model, rats underwent SNS or sham surgery; after 10 days, AP, NP or Veh (10 μl) was injected i.t. Withdrawal responses were assessed using the Randall–Selitto test. b,c, Capsaicin-induced mechanical allodynia in mice: kinetic VFF response (b) and integrated response as area under the curve (AUC) (c). d–f, CFA-evoked mechanical hyperalgesia in mice: VFF response (d), AUC (e) and half width response (f). g–i, SNS-evoked mechanical hyperalgesia in rats: neuropathic withdrawal threshold response (g), AUC (h) and half width response (i). Data are presented as mean ± s.e.m., n = 6 animals for all experiments. *P < 0.05, **P < 0.005, ***,#P < 0.001, ##P < 0.0001 compared to vehicle. Two-way ANOVA, Dunnett’s post-hoc test (b,d,g); one-way ANOVA, Dunn’s post-hoc test (c,e,f,h,i).
