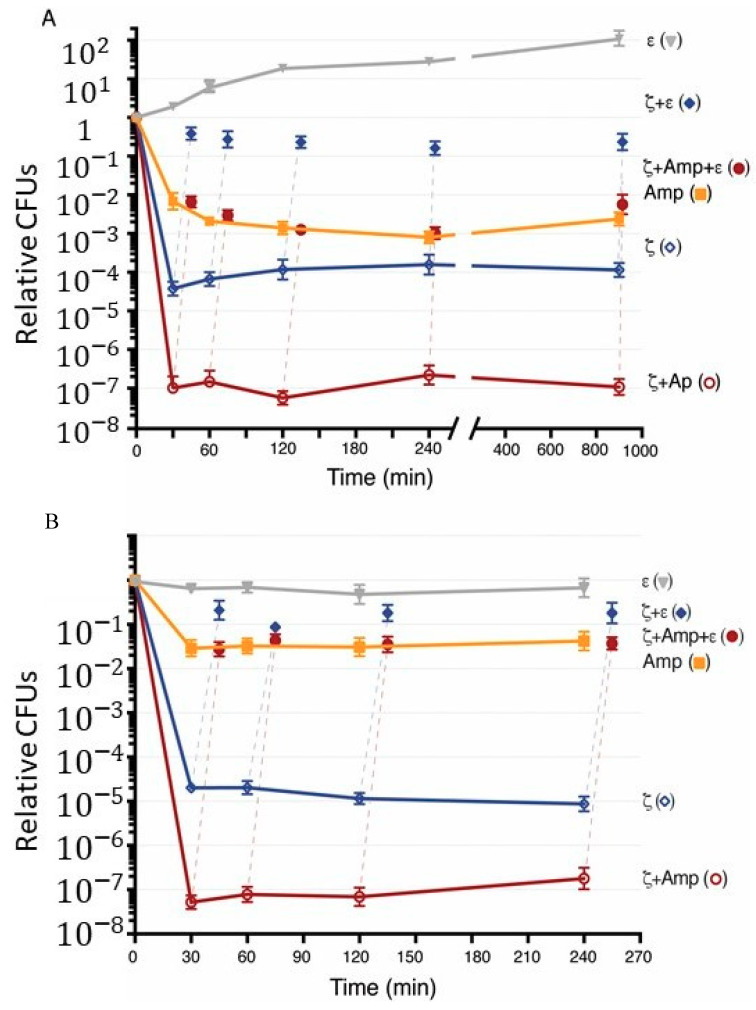
Figure 2.
Toxin ζ induces reversible dormancy, and facilitates Amp dormancy. (A), BG1125 (pCB799) cells were grown in the S7 medium, containing traces of Xyl (0.005%) at 37 °C and the cultures were divided. Then, IPTG (2 mM) to induce ζ expression, Amp (at 2× MIC, 3 μg mL−1), or both IPTG and Amp were added (0 min), and the cultures were incubated for 900 min. At various times, samples were withdrawn, centrifuged, and plated on LB agar plates to count ζ (empty blue rhomb), Amp (filled orange square), or both ζ and Amp survivals (empty purple circles). At 30, 60, 120, 240, and 900 min, aliquots were taken and 0.5% Xyl was added to induce antitoxin ε expression, and the cultures were incubated for 15 min before plating in LB agar plates containing Xyl, but lacking IPTG (filled blue rhomb) or both IPTG and Amp (filled purple circle). The vertical broken lines join the original point (ζ or ζ + Amp) with the reversed condition after ε expression. (B) An overnight culture of BG1125 (pCB799) cells grown in the S7 medium, containing traces of Xyl (0.005%) was normalized to ~1 × 109 cells mL−1 (37 °C) and the cultures were divided. Xyl (0.5%) to induce ε expression as the control was added. IPTG to induce toxin, Amp, or both IPTG and Amp was added (0 min), and the cultures were incubated for 240 min. At various times, samples were withdrawn and plated in LB agar plates lacking IPTG (empty blue rhomb), Amp (filled orange square), or both IPTG and Amp (empty purple circles). At various times, 0.5% Xyl was added to induce antitoxin expression, and the cultures were incubated for 15 min before plating. Samples were withdrawn and plated in LB agar plates containing Xyl, but lacking IPTG (filled blue rhomb) or both IPTG and Amp (filled purple circle). The vertical broken lines join the original point (ζ or ζ + Amp) with the reversed condition after ε expression. Data are shown as mean ± standard error of the mean (SEM), from >4 independent experiments.