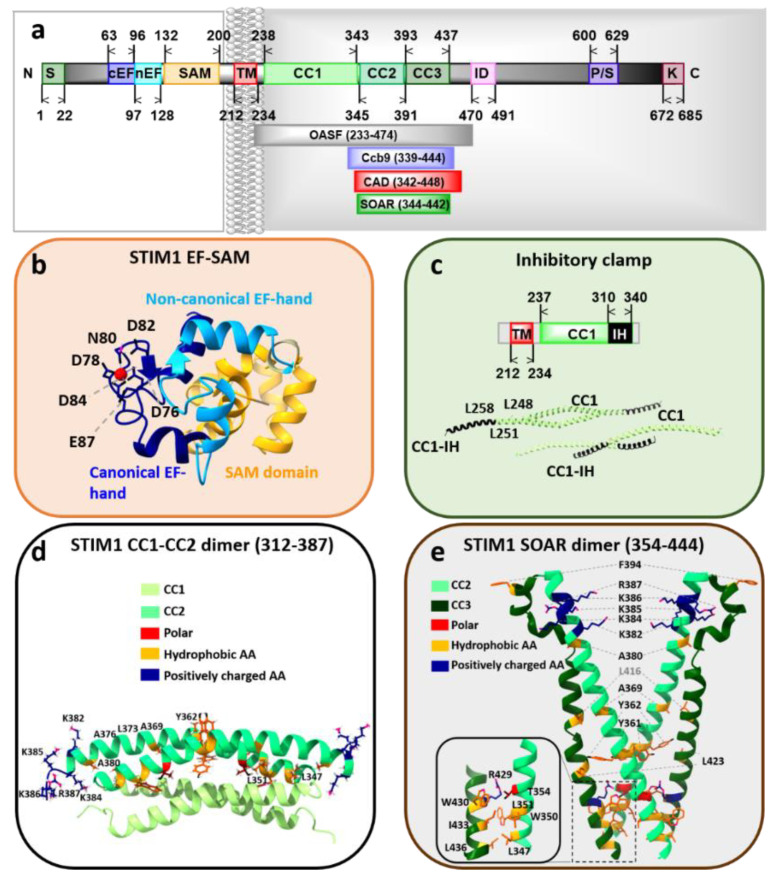
Figure 2.
The structural features of Stromal interaction molecule 1 (STIM1). (a) Scheme displays the full-length human STIM1 with highlighted regions critical for the regulation of the STIM1/Orai1 signaling cascade. Important fragments, such as OASF, Ccb9, CAD, and SOAR are further shown as insets. (b) The high-resolution EF-SAM domain structure of human STIM1 loaded with a Ca2+ ion (red sphere) is displayed. The residues with proposed Ca2+-binding ability are highlighted. (c) The crystal structure of STIM1 CC1-inhibitory helix domain with the critical residues maintaining the STIM1 quiescent state are presented. (d) The NMR structure of a STIM1 CC1α3-CC2 dimer with the two monomers coupled in an antiparallel manner. Each monomer is bent with a sharp kink between the two coiled-coil domains. (e) The crystallographic structure of the STIM1 SOAR dimer, forming a V-shape. A single monomer comprises CC2 and CC3 domains and resembles the capital letter, “R.” Residues that represent potential interaction sites within the dimer and those mediating coupling to Orai1 are highlighted. Left inset: Magnified view of amino acids involved in dimer interactions between the N-terminal portion of the first SOAR monomer and the C-terminal portion of the second SOAR monomer.