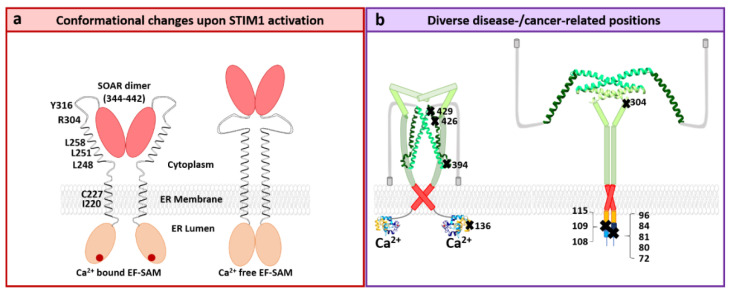
Figure 6.
STIM1 activation and diverse positions related to diseases. (a) Cartoon representation of STIM1 dimer in the inactive state (left) and activated state (right). In the inactive state, the EF-SAM domains are both loaded with Ca2+ ions and do not display any interaction. The transmembrane (TM) helices that connect the N-terminus of STIM1 to the C-terminus in the cytoplasm are spatially separated. The CC1 region displays an “inhibitory helix pocket” with important residues maintaining the STIM1 quiescent state highlighted. SOAR, comprised of residues 344–442, forms intramolecular interactions with CC1. In the activated state (right), the SOAR region decouples from CC1 and is exposed, the EF-SAM domains interact and the transmembrane helices moving closer to another (modified according to Hirve et al. [163]). (b) Diverse positions within the STIM1 protein leading to either loss of function (left) or gain of function (right). The highlighted residues represent disease-related positions that can lead to immunodeficiencies, tubular aggregate myopathy, Stormorken syndrome, York platelets syndrome, or even cancer upon mutation.