Figure 7.
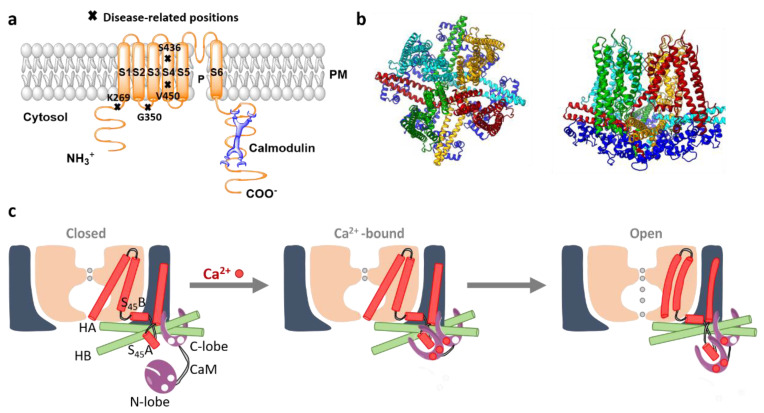
The structure and activation mechanism of SK channels. (a) The proposed structure of the SK channel with constitutively bound CaM. P represents the pore region of the channel. Moreover, residues that have been associated with the rare developmental disorder, the Zimmermann–Laband Syndrome, are highlighted. (b) The top and side view of SK4 tetramer (PDB ID 6CNM; 4 subunits colored distinct in red, yellow, light blue, green) bound to 4 CaM (dark blue). (c) The scheme depicts the stepwise gating mechanism of the SK channel. Under the Ca2+ free conditions (left panel), the SK channel remains closed. The C-lobe of CaM is constitutively associated to the channel, whereas the CaM N-lobe possesses diverse conformations due to a high level of flexibility and almost no present interaction to the channel. The binding pocket of CaM N-lobe at this stage stays closed. In the presence of Ca2+, the ions bind to the CaM N-lobe, which structurally rearranges into a more open conformation (middle panel). The latter rearrangement allows the interaction of CaM N-lobe with the S45A helix. In the following, the N-lobe pulls the S45A toward the cytosol, displacing S45B away from the pore axis (right panel). Subsequently, the S6 helical bundle expands and potentially opens the pore. Modified from [304].