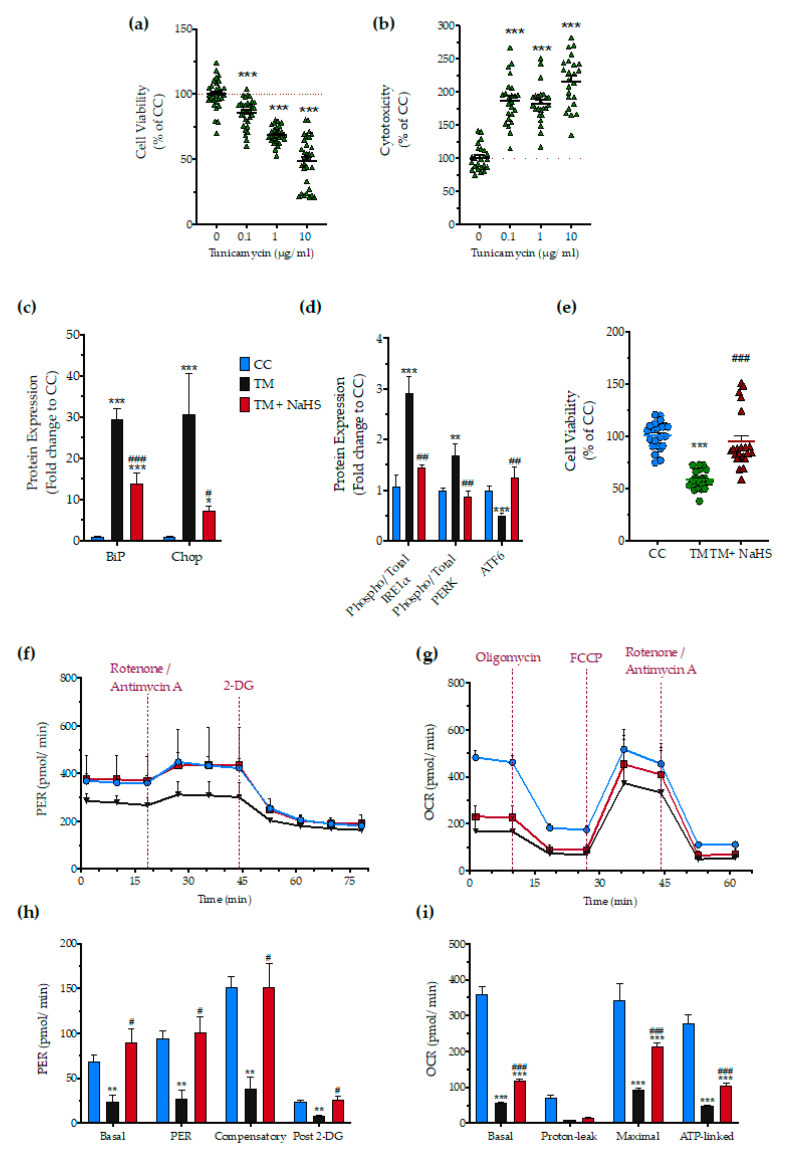
Figure 13.
No-calcium dependency in the hepatoprotective effects of NaHS under conditions of persistent ER stress. HepG2 cells serum-starved for 8 h and treated with 0, 0.1, 1, and 10 µg/mL of tunicamycin (TM) for 16 h for stressor concentration optimization. Micro-cultures were assayed for the XTT conversion (a) to assess cell viability. Cell supernatant was collected and processed for the LDH activity (b) to determine cytotoxicity. We selected the concentration of 1 µg/mL of tunicamycin for the experiments. For the subsequent experiments, HepG2 cells were treated with the vehicle or 1 µg/mL of tunicamycin, with/without 100 µM NaHS for 16 h. Following the treatment incubation, whole-cell lysate was collected and processed for protein expression of the UPR sensors-BiP (d), IRE1α (activating phosphorylation at Ser724), PERK (activating phosphorylation at Thr 980), and total ATF6 (c). We additionally quantified the expression levels of the UPR apoptotic mediator-Chop (c). Micro-cultures were assayed for XTT conversion (e) or cellular bioenergetic function using the Seahorse XFe24 Extracellular Flux Analyzer for assessing oxidative phosphorylation (f,h) and glycolysis (g,i). Each graph line and bar represent mean ± SEM from three independent experiments. Data are expressed as a percentage or fold change of the control, untreated/unstressed conditions (CC). * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001 compared to corresponding CC; # p ≤ 0.05, ## p ≤ 0.01 and ### p ≤ 0.001 compared to the corresponding TM-treated cells.