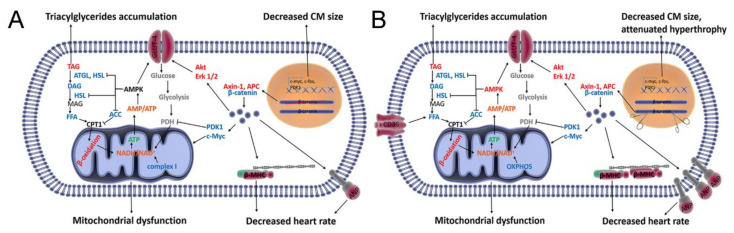
Figure 8.
Schematic representation of the canonical Wnt signaling function in regulation of cardiac metabolism under the sedentary conditions (A) and after the endurance training (B). (A) Heterozygous knockout of β-catenin inhibits canonical Wnt signaling and downregulates β-catenin target genes (c-Myc, c-Fos). This leads to decreased cardiomyocytes size. The higher levels of ANP and β-MHC may lead to the lower heart rate. Canonical Wnt signaling is involved in FA metabolism and regulation of mitochondria function via its targets c-Myc and PDK1. Decreased canonical Wnt signaling is associated with the activation of Pi3K–Akt and MAPK/Erk1/2 signaling pathways. Altogether, this causes the inhibition of lipolysis and the activation of glucose uptake in hearts of WT/CKO mice. Activation of β-oxidation and glucose oxidation along with lower activity of complex I lead to the accumulation of NADH, which promotes mitochondrial dysfunction. (B) The lower level of canonical Wnt signaling attenuates the cardiomyocytes hypertrophy. Adaptation of WT/CKO mice to the endurance training is accompanied by activation of AMPK and a stronger activation of pre-activated Pi3K–AKT and MAPK/Erk1/2 signaling pathways. Increased AMPK leads to the inhibition of lipolysis and activation of β-oxidation. Activation of AMPK, Pi3K–AKT, and MAPK/Erk1/2 signaling pathways stimulates FA and glucose uptake. Downregulation of canonical Wnt signaling reduces mitochondria biogenesis and OXPHOS activity in the heart during adaptation to the endurance training. Decreased OXPHOS protein level and activity along with enhanced β-oxidation further exacerbate mitochondrial dysfunction. Notes: Red—observed increase; blue—observed decrease; orange—possible increase; green—possible decrease; black—remain unchanged relative to relevant WT/WT mice.