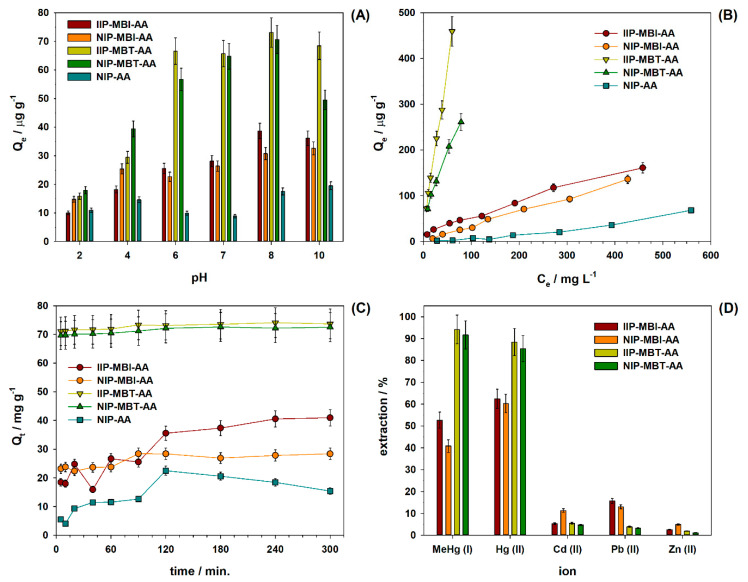
Figure 4.
(A) Effect of pH on sorption of MeHg+ on IIP–MBI–AA, NIP–MBI–AA, IIP–MBT–AA, NIP–MBT–AA, and NIP–AA; 3 mg of polymeric material, [MeHg+] = 100 µg L−1, volume = 2.0 mL, shaking time = 2 h, room temperature. (B) The effect of MeHg+ initial concentration on the adsorption of MeHg+ on IIP–MBI–AA, NIP–MBI–AA, IIP–MBT–AA NIP–MBT–AA, and NIP–AA; 3 mg of polymeric material, [MeHg+] = 25–800 µg L−1, pH = 8, volume = 2.0 mL, shaking time = 2 h, room temperature. (C) Kinetics of MeHg+ adsorption on IIP–MBI–AA, NIP–MBI–AA, IIP–MBT–AA NIP–MBT–AA, and NIP–AA; 3 mg of polymeric material, [MeHg+] = 100 µg L−1, pH = 8, shaking time = 5–300 min, volume = 2.0 mL, room temperature. (D) The selectivity profiles of the IIPs and NIPs for binary mixtures containing MeHg+ and potential interferents. In all experiments n = 3.