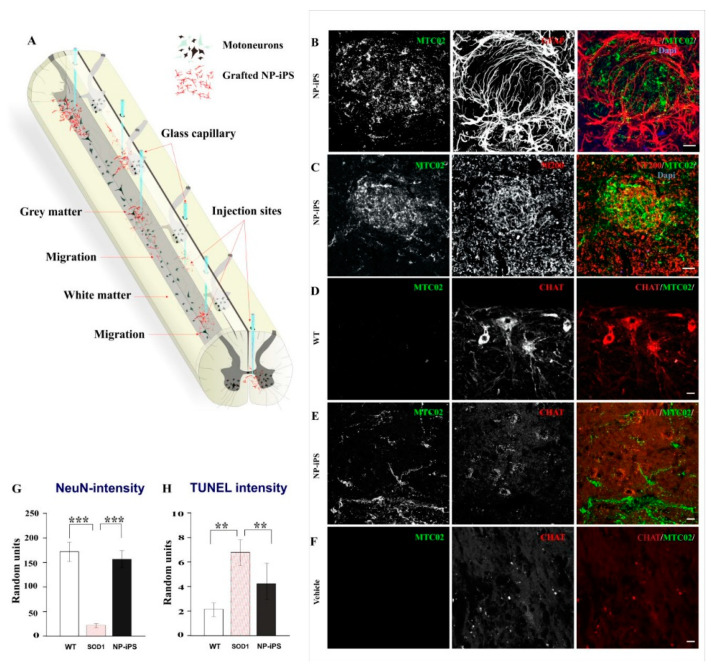
Figure 4.
Survival and engraftment of NP-iPS. Schematic diagram shows a map of intraspinal injection sites (via the glass capillary tubes) and distribution of the grafted cells within the spinal cord (A). Intraspinally transplanted NP-iPS engrafted into the host tissue at the site of injection (B), Engrafted cells also preserved the expression of host neurofilament (C). Compared to WT rats (D), cell-treated animals (E) had weaker acetylcholine transferase (CHAT) staining, even though NP-iPS engrafted in the close vicinity to host MNs (E); however, NP-iPS-treated rats still maintained CHAT-positive cells (E) compared with vehicle-treated animals, where the CHAT staining was almost undetectable (F). A neuroprotective effect evaluated by NeuN fluorescence intensity revealed a significantly higher fluorescence in the ventral horns of NP-iPS-treated superoxide dismutase (SOD1)-rats compared with the vehicle-treated SOD1 littermates (G). TUNEL staining confirmed less apoptosis in NP-iPS-treated SOD1-rats compared with the vehicle-treated SOD1 littermates (H). Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) staining. Differences in the groups were analyzed by one-way ANOVA, p < 0.01 (**), p < 0.001 (***). Scale bars = 20 µm.