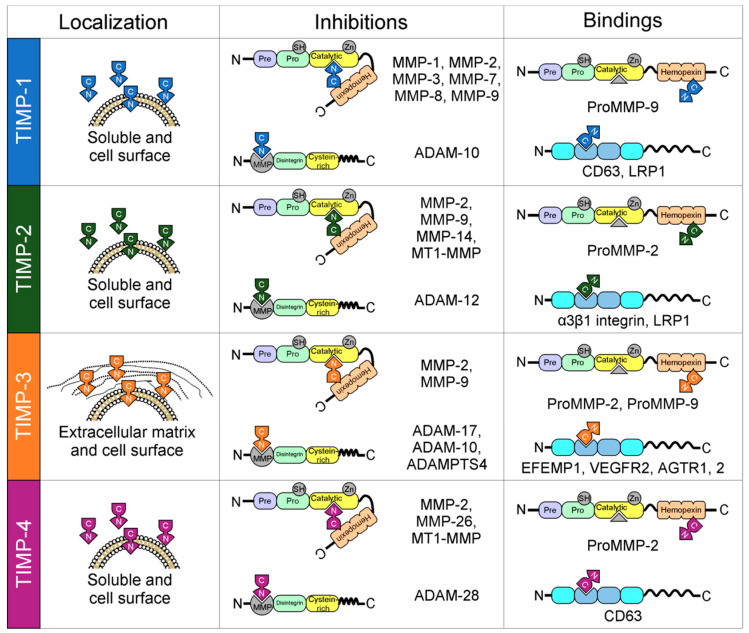
Figure 1.
Localization and interactions between tissue inhibitors of matrix metalloproteases (TIMPs) and matrix metalloproteases (MMPs). All TIMPs are secreted, but only TIMP-3 is incorporated into the matrix. Structurally, TIMPs are comprised of two domains that pack side-by-side (N-terminal and C-terminal domains). The N-terminal domain is sometimes referred to as the “inhibitory domain”. TIMP-1 inhibits MMP-1–3 and MMP-7–9; TIMP-2 inhibits MMP-2, MMP-9, MMP-14, and membrane-type matrix metalloproteases 1 (MT1-MMP); TIMP-3 inhibits MMP-2 and MMP-9; and finally, TIMP-4 inhibits MMP-2, MMP-26, and MT1-MMP. In addition, TIMPs interact with the proforms of MMPs in a non-inhibitory manner, and they also have functions independent of MMP inhibition by directly binding to cell surface receptors (TIMP-1 to CD63; TIMP-2 to α3β1integrin and LRP1; TIMP-3 to EFEMP1, VEGFR2 and AGTR1,2; and TIMP-4 to CD63).