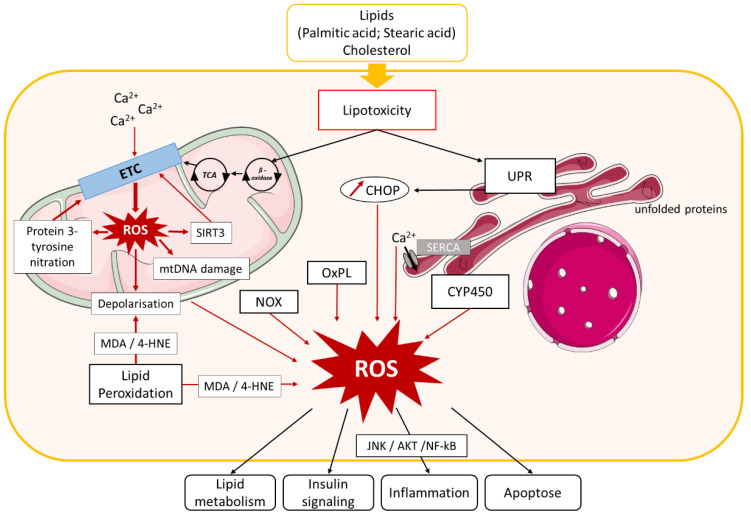
Figure 2.
Role of oxidative stress in NAFLD. Lipid and cholesterol accumulation in hepatocytes causes lipotoxicity, resulting in activation of different pathways involved in oxidative stress. Lipid entry and alteration of mitochondrial Ca2+ homeostasis cause electron transport chain (ETC) dysfunction that leads to mitochondrial reactive oxygen species (ROS) production, which in turn induces Sirtuin (SIRT)3 expression, protein 3-tyrosine nitration, mitochondrial DNA damage, and membrane depolarization. SIRT3 and protein nitration also cause ETC dysfunction and exacerbate mitochondrial ROS production. Mitochondrial biogenesis dysfunction additionally induces ROS production. Lipotoxicity activates endoplasmic reticulum (ER) stress, leading to ROS production by (i) unfolded protein response (UPR) activation, which stimulates C/EBP homologous protein (CHOP) expression and (ii) Ca2+ homeostasis dysfunction. ROS generation in cytoplasm is induced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzyme activation, oxidized phospholipids (OxPL), several cytochrome p450 enzymes (CYP450), and lipid peroxidation. ROS accumulation in hepatocytes leads to impairment of several pathways involved in NAFLD development, such as lipid metabolism, insulin signaling, inflammation, and apoptosis. Other abbreviations: mtDNA, mitochondrial DNA; TCA, tricarboxylic acid cycle; MDA, malondialdehyde; 4-HNE, 4-hydroxynonenal; SERCA, ER calcium pump sarco/endoplasmatic reticulum Ca2+-ATPase; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-kappa B.