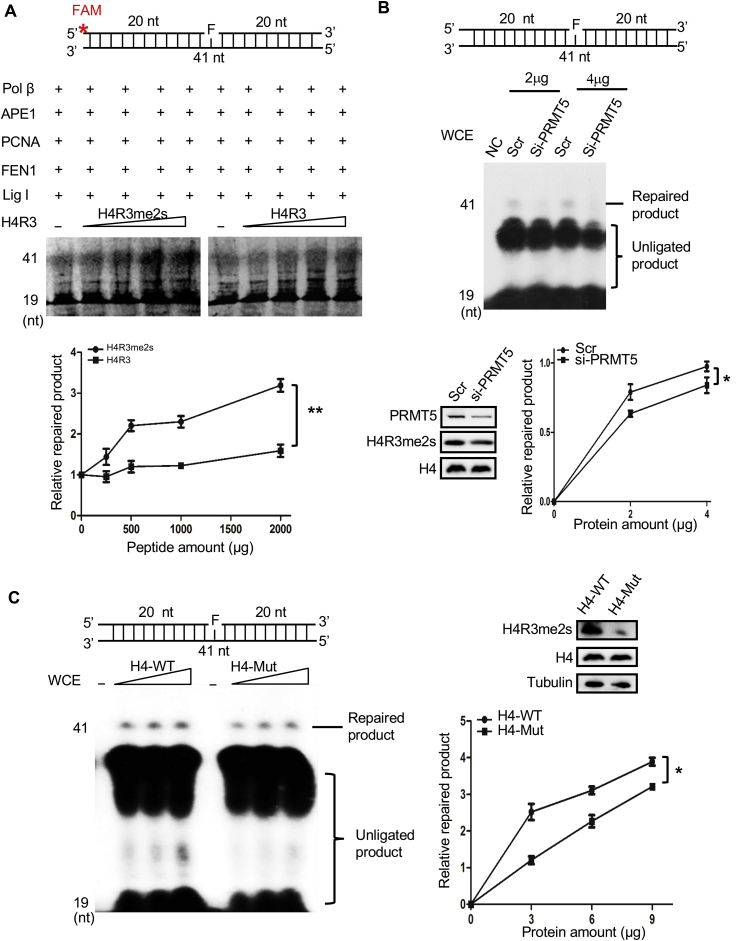
Fig. 6.
H4R3me2s enhances LP-BER efficiency. Effect of H4R3me2s on LP-BER efficiency. The top parts of each panel show schematic structures of the corresponding DNA substrates. (A) Purified BER proteins and increasing amounts (250 ng, 500 ng, 1000 ng, 2000 ng) of H4R3me2s or H4R3 peptides were subjected to a reconstituted LP-BER assay in vitro. The substrates were incubated with proteins at 37 °C for 30 min. Representative PAGE gels are shown with arrows denoting the repaired product and the unligated product. The graph represents the quantification of the PAGE results in the image, and the values represent the mean ± SD of three independent assays (**P < 0.01, Student's t-test). (B) HEK293T cells transfected with PRMT5 siRNA or scrambled siRNA (Scr) were treated with 1 mM H2O2 for 30 min, and the whole-cell extracts were then collected for reconstituted LP-BER and Western blot assays. Representative PAGE gels are shown with arrows denoting the repaired product and the unligated product. Western blot analysis was performed with anti-PRMT5, anti-H4R3me2s and anti-H4 antibodies. The graph represents the quantification of the PAGE results in the image, and the values represent the mean ± SD of three independent assays (*P < 0.05, Student's t-test). (C) HeLa cells transfected with Flag-tagged WT H4 or Flag-tagged mutant H4 R3Q were treated with 1 mM H2O2 for 30min, and whole-cell extracts were then collected for reconstituted LP-BER and Western blot assays. The left panel shows representative PAGE gels. The level of H4R3me2s modification was detected by Western blotting, with Tubulin used as the loading control. The graph represents the quantification of the PAGE results in the image, and the values represent the mean ± SD of three independent experiments (*P < 0.05, Student's t-test).