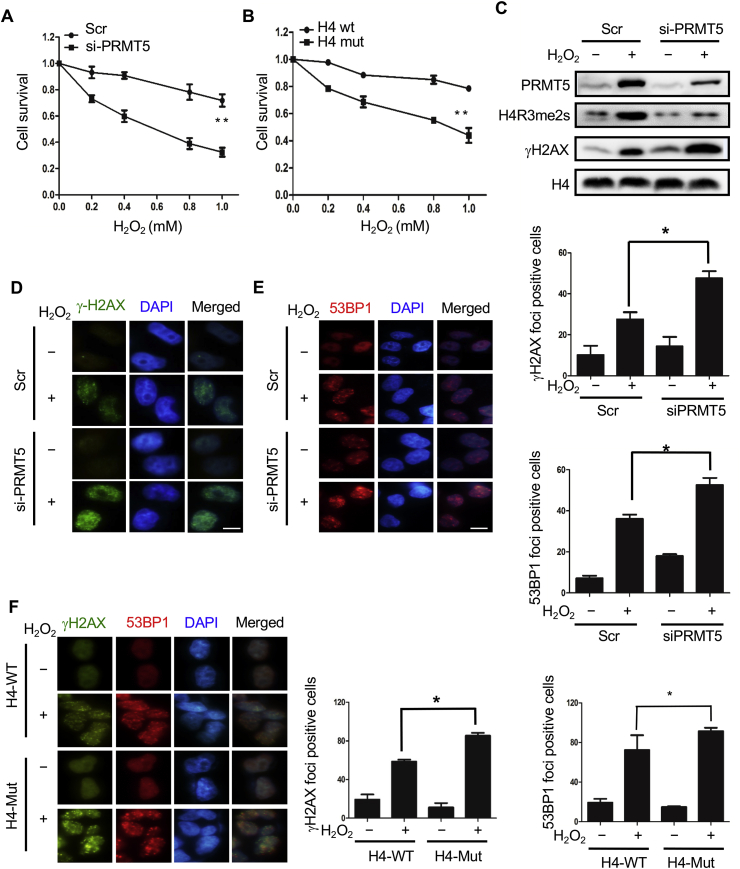
Fig. 7.
The H4R3me2s modification is essential for DNA repair. (A, B) H2O2 sensitivity assays. Cells (1 × 105 per well) were plated in triplicate in six-well plates. Cells transfected with PRMT5 siRNA or scrambled siRNA (A) or Flag-tagged WT H4 or Flag-tagged mutant H4 R3Q (B) were treated with different doses of H2O2 for 30 min, washed with PBS and cultured in fresh media for 2 days. The number of viable cells in every well was determined following the trypsinization of the cells and counting with a cell counter (Countstar IC1000). The control growth ratio was calculated based on the numbers of treated/untreated cells. The data represent the mean ± SD from triplicate wells. Three independent experiments were performed. (C) HeLa cells transfected for 48 h with PRMT5 siRNA or scrambled siRNA were treated with 1 mM H2O2 for 30 min, and cells were then lysed and subject to Western blotting to detect H4R3me2s and γH2AX levels. (D, E) HeLa cells transfected for 48 h with PRMT5 siRNA or scrambled siRNA were treated with 1 mM H2O2 for 30 min γH2AX foci (D) and 53BP1 foci (E) in the cells were detected via immunofluorescence analysis as previously described. Fifty random regions were examined at 400× magnification. Nuclei containing ≥1 foci were counted as positive for focus formation, and the percentage of positive cells was calculated and plotted. The data represent the mean ± SD of three independent experiments. *P < 0.05, Student's t-test. Scale bars, 10 μm. (F) HEK293T cells transfected for 48 h with Flag-tagged WT H4 or Flag-tagged mutant H4 R3Q were treated with 1 mM H2O2 for 30 min. Cells were fixed and stained with antibodies against γH2AX (green) and 53BP1 (red). DNA was stained with DAPI (blue). Left histogram, γH2AX focus analysis; right histogram, 53BP1 focus analysis. The data represent the mean ± SD of three independent experiments. *P < 0.05, Student's t-test. Scale bars, 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)