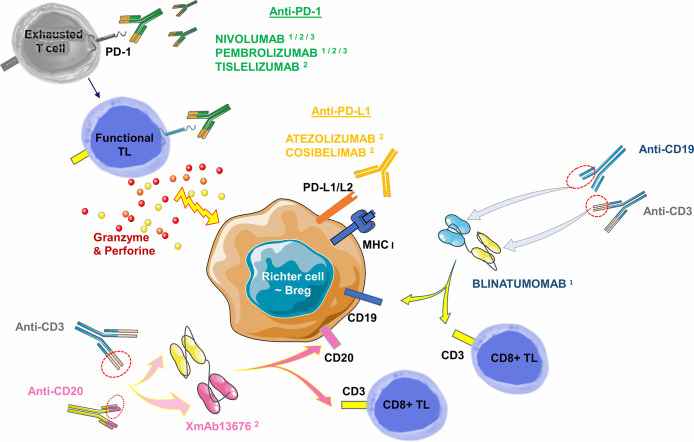
Figure 7.
Monoclonal antibodies targeting the PD-1/PD-L1 axis (checkpoint inhibitors) and bispecific antibodies commercially available or in published/ongoing clinical trials in RS. Monoclonal antibodies targeting the PD-1/PD-L1 axis can be divided into two groups: i) against PD-1 receptor and ii) against its ligands PD-L1 or PD-L2. Anti-PD-1 antibodies are mainly represented by Nivolumab: a full human IgG4, and Pembrolizumab: a humanized IgG4. Tislelizumab (BGB-A317) is a new humanized IgG4 anti-PD-1 antibody. Anti-PD-L1 antibodies are mainly represented by Atezolizumab, a humanized antibody. Cosibelimab (TG-1501) is a novel, fully humanized anti-PD-L1 antibody. Bispecific antibodies are designed to direct cytotoxic TLs expressing CD3 towards B-cells expressing CD19 (Blinatumomab) or CD20 (XmAb13676). Breg, Regulatory B Lymphocyte; MHC, Major Histocompatibility Complex; PD-1, Programmed Death 1; PD-L1, Programmed Death Ligand 1; PD-L2, Programmed Death Ligand 2; TL, T Lymphocyte. 1: commercially approved in other hemopathies (relapsed Hodgkin Lymphoma for Nivolumab and Pembrolizumab, relapsed/refractory Acute B Lymphoid Leukemia for Blinatumomab). 2: clinical trials published in RS. 3: clinical trials underway in RS.