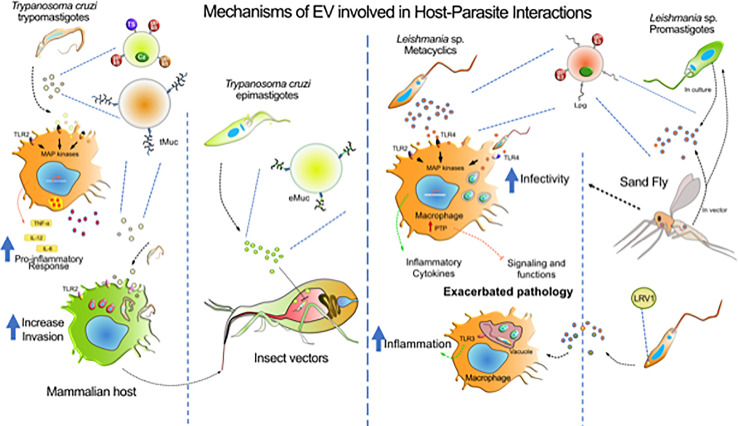
Figure 2.
Mechanisms of EV involved in host-parasite interactions. The diagram represents the major contributions of EVs from T. cruzi, Leishmania sp. and host cells during the infection by these parasites. T. cruzi release heterogeneous populations of EVs containing surface mucin-like glycoproteins (tMuc and eMuc), GPI anchored proteins (GP63 protease, GP85, and TS superfamily members) and soluble proteins (cruzipain, Cz), which interact with macrophages through TLR2 receptors which activate MAP kinases and induce a pro-inflammatory response (TNF-α, IL-12, and IL-6). Macrophages also release EVs that might affect the host immune system. The TLR2—T. cruzi EVs interaction enhances further parasite invasion. In the insect vector, the parasite epimastigote EVs delays translocation of parasites through intestine. Leishmania metacyclics release EVs enriched with GP63 and LPG, that could interact with macrophages via TLRs and, depending on the species, to induce pro- and anti-inflammatory responses. GP63-enriched EVs were found to induce host protein tyrosine phosphatases concurring to alter macrophage signaling and microbicidal functions. However, receptors involved in EVs recognition concurring to augment infection of phagocytes is still not identify. Leishmania virus (LRV1) incorporate within Leishmania exosomes was found to induce myeloid cells (macrophage, neutrophil) inflammasome in a TLR-dependent fashion concurring to exacerbate skin pathology. Leishmania exosomes are released in the gut of the sand Fly vector and have been found to be co-inoculated with promastigotes during blood meal. Revealing that this co-inoculation can exacerbate myeloid cells infection and skin pathology development.