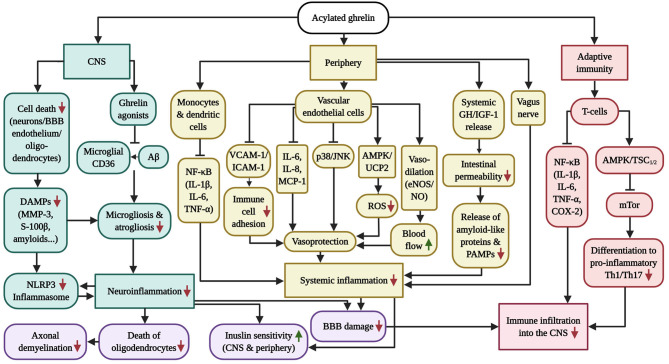
Figure 2.
Overview of the anti-inflammatory capabilities of GHS-R1α receptor activation. AD and PD are characterized by chronic systemic inflammation, which includes micro-/astrogliosis and inflammasome activation following the accumulation of amyloids and DAMPs in the CNS, vagus nerve and intestinal (microbiome) inflammation in the periphery as well as pathologic CD4+ T-cell infiltration into the brain, which is exacerbated by the inflammation-driven injury of the BBB and vasculature. While AG has successfully prevented neuroinflammation in AD and PD models, the diagram further illustrates the beneficial effects of AG on inflammasome induction, peripheral inflammation and adaptive immunity in other inflammatory disease models, which culminate in vascular protection as well as enhanced blood flow, BBB stability, insulin sensitivity, oligodendrocyte survival and axonal myelination. Of note, GHS-R1α does not appear to be expressed by microglia, suggesting that the anti-inflammatory benefits of AG in the CNS are indirect. Ghrelin agonists offer the additional benefit of blocking microglial CD36, thus inhibiting Aβ-elicited inflammation.