FIGURE 2.
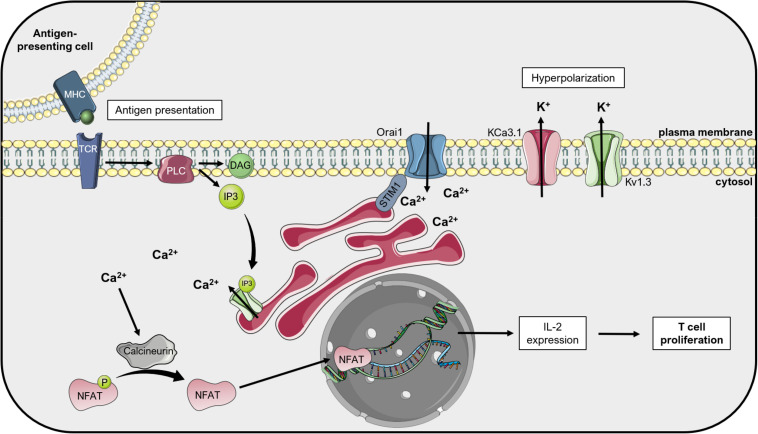
Kv1.3 regulates T lymphocyte proliferation: the «membrane potential model». Upon activation of T cell receptors (TCR) by antigen-presenting cells, phospholipase C (PLC) cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 activates the IP3 receptor (IP3R) on the endoplasmic reticulum (ER), which releases Ca2+ into the cytosol. Ca2+ depletion from the ER lumen leads to conformational changes of the ER-resident protein STIM1, which couples the ER to the plasma membrane and activates Orai1, a calcium release-activated Ca2+ channel (CRAC). The following increased Ca2+ concentration in the cytosol activates the phosphatase Calcineurin, which dephosphorylates the Nuclear Factor of Activated T cells (NFAT). This transcription factor translocates to the nucleus and activates the transcription of interleukin-2 (IL-2), thus inducing T cell proliferation. The calcium-activated potassium channel KCa3.1 and the voltage-dependent Kv1.3 alternately open during this process and give rise to the potassium efflux that hyperpolarizes the plasma membrane, thus providing the driving force for sustained Ca2+ influx (Teisseyre et al., 2019). This figure was created using images from Servier Medical Art (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.