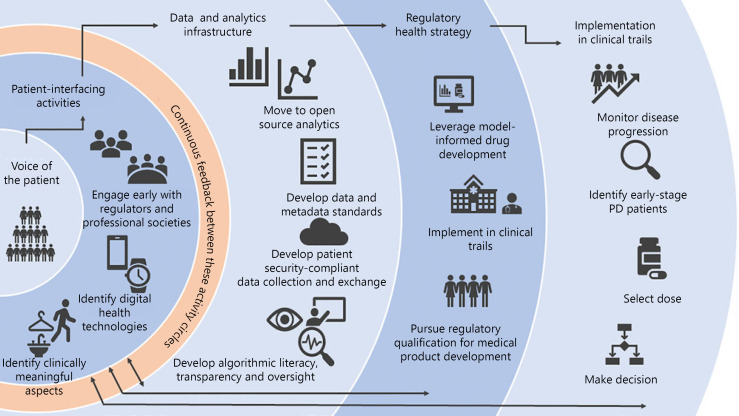
Fig. 1.
Illustration of the multifaceted aspects of advancing DHT for use in PD clinical trials. Left to right: flow scheme that starts with the patient, then advances to data-driven rigor and compliance, along with regulatory acceptance and adoption in clinical trials. The original focus on the voice of the patient facilitates the identification of clinically meaningful concepts of interest. Data standardization and definition of sources of variability are key to early steps to engage regulatory agencies. Implementation in PD trials is envisioned (right) when the steps to the left are followed.