Figure 2.
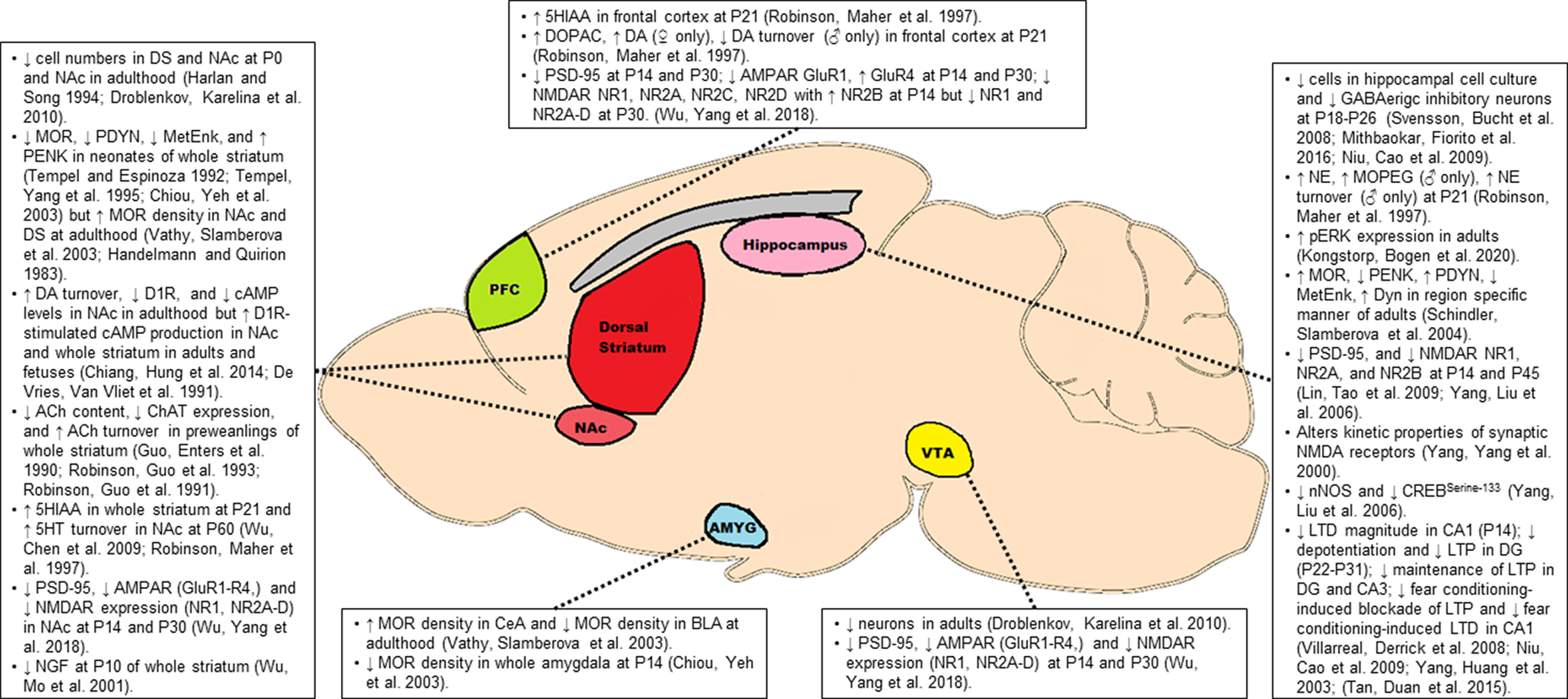
Molecular alterations in the key brain regions associated with reward. Opioids freely cross the placenta allowing access to the fetal central nervous system where they may reduce neuronal numbers and induce persistent disruptions in opioidergic, catecholaminergic, glutamatergic, and cholinergic signaling among other biochemical alterations. These molecular alterations in key nodes of the mesocorticolimbic pathway may contribute to the enhanced addictive phenotype observed in offspring with POE. See text for a more detailed description of some of these molecular alterations. 5HT, 5-hydroxytryptamine (serotonin); 5HIAA, 5-hydroxyindoleacetic acid (serotonin metabolite); Ach, acetylcholine; AMPAR, AMPA receptor; Amyg, amygdala; BLA, basolateral amygdala; CeA, central amygdala; cAMP, cyclic adenosinemonophosphate; ChAT, choline acetyltransferase; CREB, cAMP response element-binding protein; DG, dentate gyrus; DA, dopamine; D1R, dopamine receptor D1; DOPAC, 3,4-dihydroxyphenylacetic acid (dopamine metabolite); DS, dorsal striatum; Dyn, dynorphin; LTD, long-term depression; LTP, long-term potentiation; MetEnk, met-enkephalin; MOR, μ-opioid receptor; NE, norepinephrine; MOPEG, 3-methoxy-4-hydroxyphenylglycol (NE metabolite); NAc, nucleus accumbens; NGF, nerve growth factor; NMDAR, NMDA receptor; nNOS, neuronal nitric oxide synthase; PDYN, prodynorphin; PENK, proenkephalin; pERK, phosphorylated ERK; PFC, prefrontal cortex; P, postnatal day; PSD-95, postsynaptic density protein 95; VTA, ventral tegmental area.
