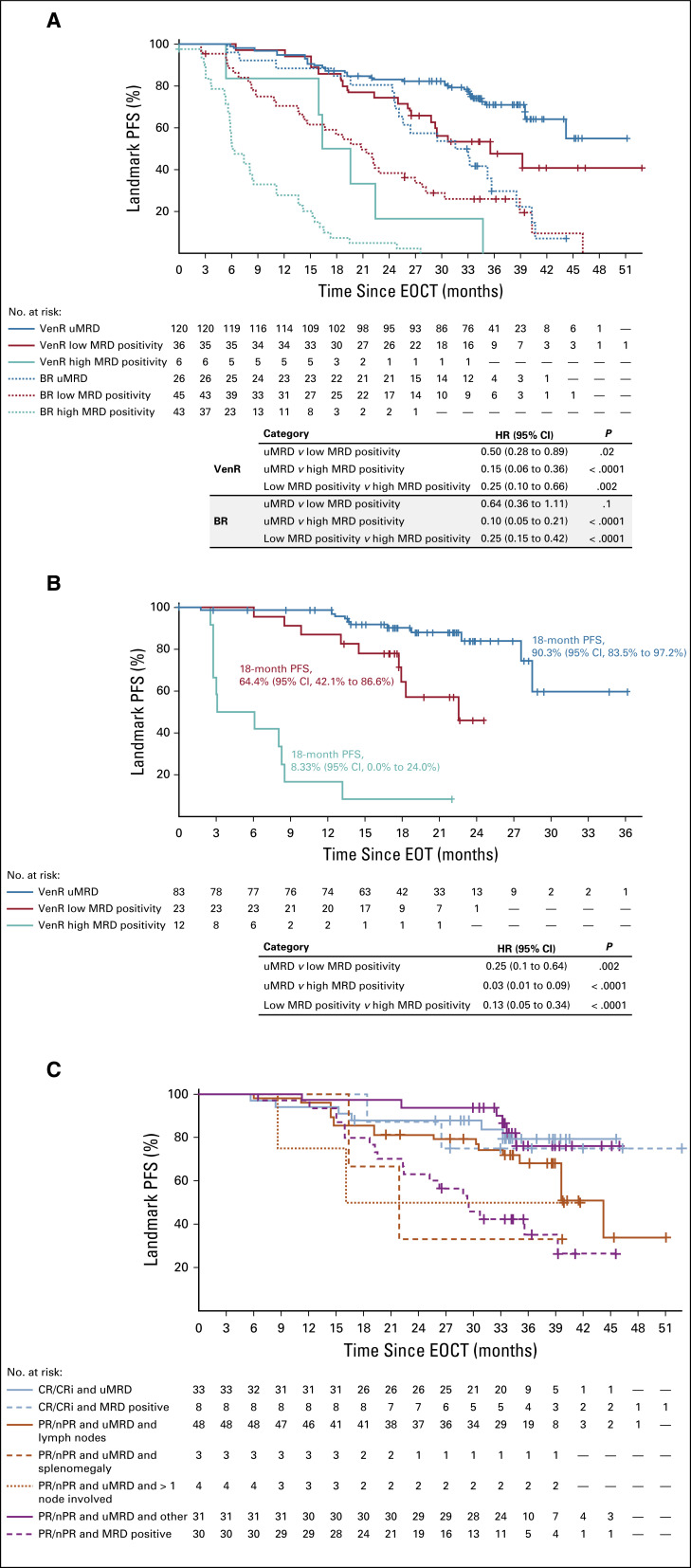
FIG 2.
Landmark Kaplan-Meier analyses. (A) Progression-free survival (PFS) from end of combination therapy (EOCT) in both study arms based on minimal residual disease (MRD) status at EOCT. (B) PFS from end of treatment (EOT) in patients in the venetoclax plus rituximab (VenR) arm who completed 2 years of venetoclax, based on MRD status at EOT (excludes two patients who completed venetoclax but experienced disease progression before MRD measurement). (C) PFS from EOCT in the VenR arm based on MRD status at EOCT and involvement of lymph nodes. BR, bendamustine plus rituximab; CR, complete response; CRi, complete response with incomplete marrow recovery; nPR, nodular partial response; PR, partial response; uMRD, undetectable minimal residual disease.