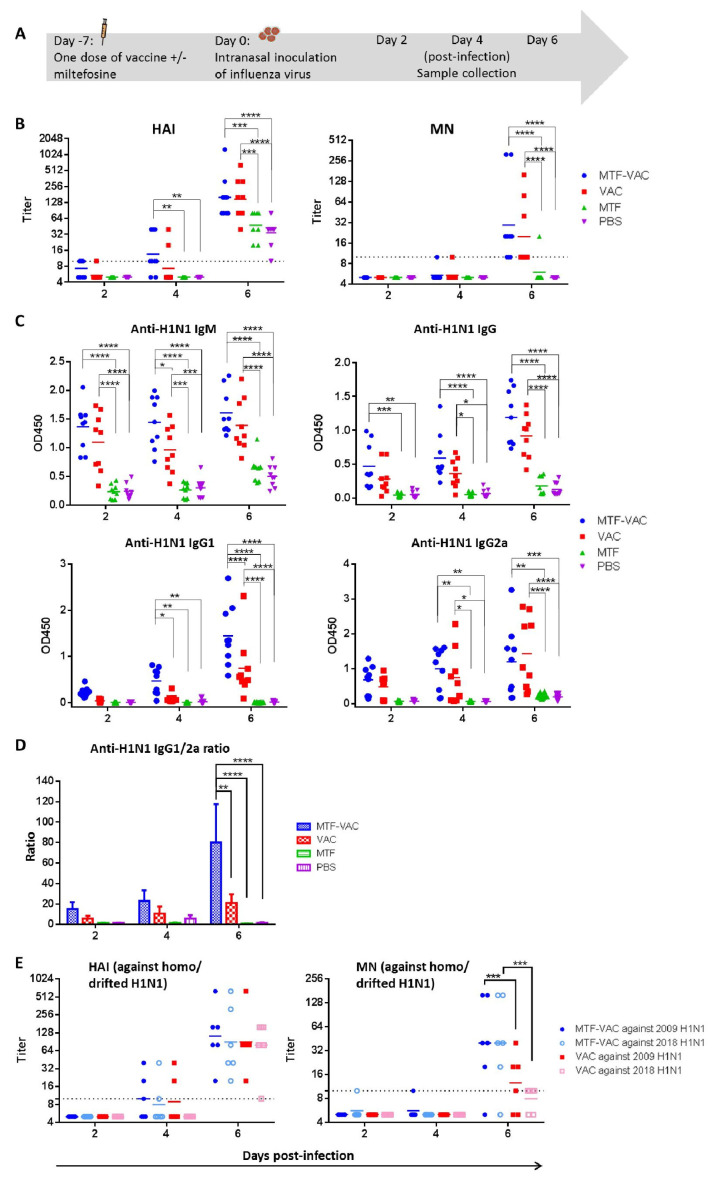
Figure 2.
The effect of a single dose of vaccine with or without miltefosine on the antibody titers administrated 7 days before influenza virus challenge. (A) Schedule of influenza vaccination, virus challenge, and sample collection. (B) Hemagglutination inhibition assay (HAI) titers and micro-neutralization assay (MN) titers of serum samples post-infection. (C) Anti-H1N1 IgM, anti-H1N1 IgG, anti-H1N1 IgG1, and anti-H1N1 IgG2a antibody levels determined by ELISA. (D) Anti-H1N1 IgG1/G2a ratio. (E) HAI titers and MN titers of serum samples taken post-infection, assessed in vitro against homologous and antigenically-drifted H1N1 strains. MTF-VAC, miltefosine (0.2 mg) + vaccine group; VAC, vaccine only group; MTF, miltefosine (0.2 mg) only group; PBS, PBS only group. Short solid lines indicate geometric means of titers, and long dashed lines indicate the detection limit of HAI and MN assays. Data collected from 9 mice per group (3 independent experiments) for (B–D), and 6 mice per group (2 independent experiments) for (E). * for p < 0.05; ** for p < 0.01; *** for p < 0.001; **** for p < 0.0001, calculated by two-way ANOVA followed by a Tukey’s multiple comparison test.