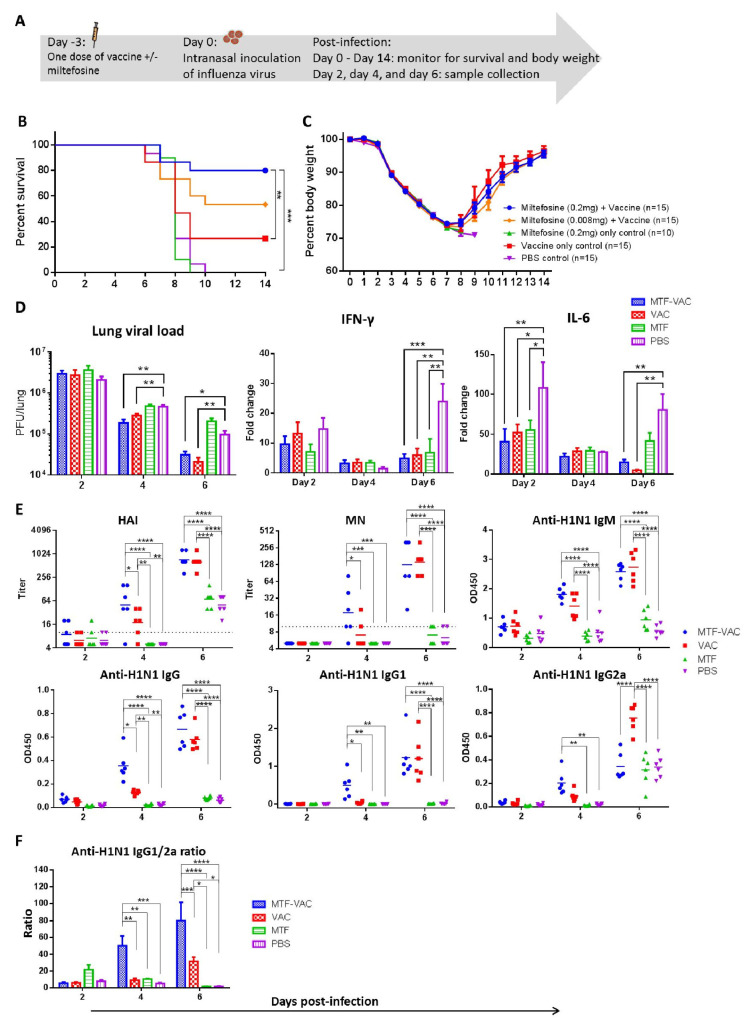
Figure 3.
The effect of a single dose of vaccine on mice administrated 3 days before influenza virus challenge. Mice were vaccinated intraperitoneally with a split-virion influenza vaccine with or without miltefosine in 200 µL. On the day of the virus challenge, 10LD50 of mouse-adapted A(H1N1)pdm09 virus was inoculated through the intranasal route in 40 µL. (A) Schedule of influenza vaccination, virus challenge, and sample collection. (B) Survival curve of mice. (C) Body weight curve of mice. (D) Pulmonary viral load (n = 6 per group) and fold change of cytokine mRNA levels in lung tissue post-infection (n = 3 per group). (E) HAI titers and MN titers of serum samples post-infection; anti-H1N1 IgM, anti-H1N1 IgG, anti-H1N1 IgG1, and anti-H1N1 IgG2a antibody levels determined by ELISA (n = 6 per group). (F) Anti-H1N1 IgG1/G2a ratio (n = 6 per group). MTF-VAC, miltefosine (0.2 mg) + vaccine group; VAC, vaccine only group; MTF, miltefosine (0.2 mg) only group; PBS, PBS only group. Data collected from 10–15 mice (3 independent experiments) for survival experiments. Log–rank (Mantel–Cox) test was used for survival comparison; multiple t-tests were used for body weight comparisons. Pulmonary viral load was determined by plaque assay and compared through multiple t-tests. Cytokine levels were assessed by RT-qPCR. Relative expression levels of target genes were normalized by β-actin expression level and fold change was calculated by comparing to naïve mice. Statistical comparisons were conducted by two-way ANOVA followed by a Tukey’s multiple comparison test. Short solid lines indicate geometric means of titers, and long dashed lines indicate the detection limit of HAI and MN assays. All antibody titer comparisons were conducted by two-way ANOVA followed by a Tukey’s multiple comparison test. * for p < 0.05; ** for p < 0.01; *** for p < 0.001; **** for p < 0.0001. Error bars represent standard error of the mean (SEM).