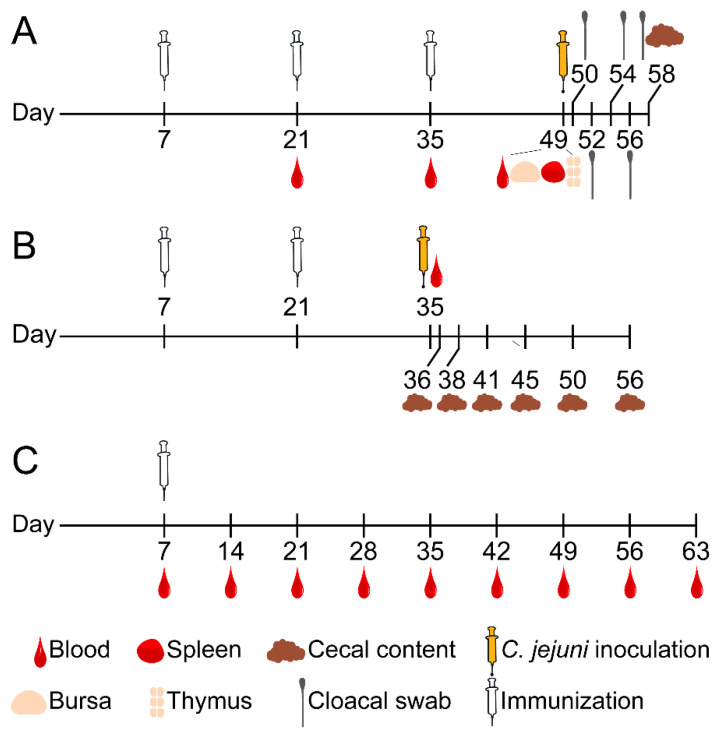
Figure 1.
Diagram of three chicken immunization trials. For each trial (20–30 SPF chickens per group), each chicken in the treatment group was immunized with 100 μg of Ent–KLH conjugate emulsified with MONTANIDE™ RANGE adjuvant (Seppic, Paris) via intramuscular injection (inner thigh) while the chicken in control group was immunized with PBS solution (0.01 M, pH 7.2) emulsified with the same adjuvant. (A) Chickens (25 birds per group) received three immunizations at age of 7, 21 and 35 days, respectively. Blood samples were collected 14 days after each of immunizations. Each chicken was weighed weekly. Nine chickens of each group were euthanized at 49 days old; major immune organs (bursa, spleen and thymus) and cecal contents were collected from each chicken as well. The remaining 16 chickens of each group were orally challenged with C. jejuni (104 CFU per chicken) two weeks after the last immunization. Cloacal swabs were collected from each chicken every 1–2 days post inoculation and cecal contents were also collected upon termination for CFU enumeration. (B) Chickens (30 bird per group) received two immunizations at age of 7 and 21 days, respectively. Blood samples were collected 14 days after the second immunization. All chickens were orally challenged with C. jejuni (104 CFU per chicken) two weeks after the second immunization. Five chickens in each group were euthanized at indicated age post challenge and cecal contents were collected from each chicken for CFU enumeration. (C) Chickens (20 birds per group) only received single immunization at age of one week old. Blood samples were collected immediately prior to immunization and weekly after the immunization until the chickens were euthanized at age of 63 days.