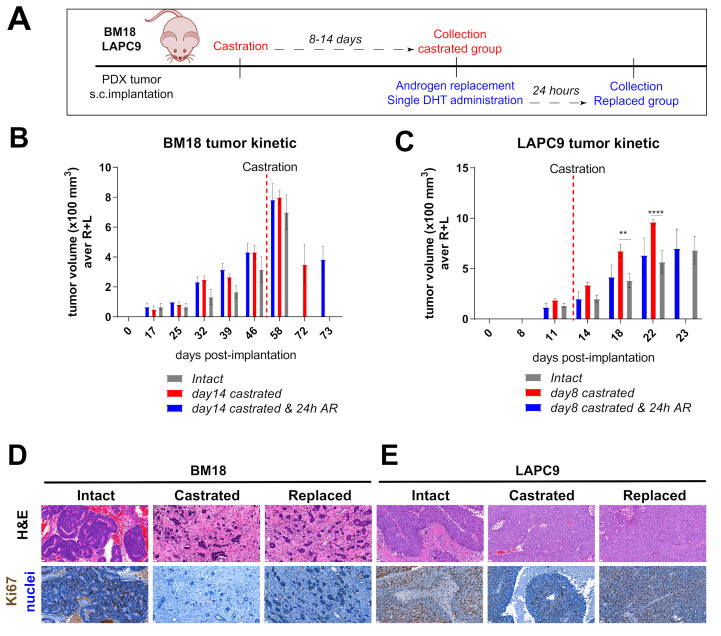
Figure 1.
In vivo tumor growth properties of androgen-dependent BM18 versus androgen-independent patient-derived xenograft (PDX) models. (A) Scheme of in vivo BM18 and LAPC9 experiments, including the timeline of castration, androgen replacement (single dihydrotestosterone (DHT) administration) and collection of material for transcriptomic analysis. (B) BM18 PDX tumor growth progression in time. Groups: (1) intact tumors (collected at max size, n = 3), (2) castrated (day 14, n = 4) and (3) castrated, followed by testosterone readministration (castrated-testosterone) (day 15 since castration and 24 h since the androgen receptor (AR), n = 3). R; right tumor, L; left tumor per animal. (C) LAPC9 PDX tumor growth progression in time. Groups: (1) intact tumors (collected at max size, n = 3), (2) castrated (day 8, n = 4) and (3) castrated, followed by testosterone readministration (castrated-testosterone) (day 9 since castration and 24 h since AR, n = 3). Tumor scoring was performed weekly by routine palpation; values represent average calculations of the tumors of all animals per group (considering 2 tumors, left, L, and right, R, of each animal). Error bars represent SEM, calculated considering the no. of animals for each time point. Ordinary two-way ANOVA with Tukey’s multiple comparison correction was performed, p ˂ 0.01 (**) and p ˂ 0.0001 (****). (D) Histological morphology of BM18 and (E) LAPC9 (from intact, castrated and androgen-replaced hosts), as assessed by Hematoxylin and Eosin staining (H&E, top). Scale bars: 20 μm, and proliferation marker Ki67 protein expression (bottom panel).