Abstract
Aims
In patients with a history of chronic alcohol abuse, neurocognitive disorders (NCD) are not uncommon. The current study aimed to explore the course of cognitive performance, as measured by the Montreal Cognitive Assessment (MoCA), and everyday cognitive functioning, as measured by the Patient Competency Rating Scale (PCRS), in a large group of patients with alcohol use disorder (AUD) admitted to the Center of Excellence for Korsakov and Alcohol-related Cognitive Impairments.
Methods
A multiple time-series design was used, in which the MoCA was administered at three time points of assessment, and the PCRS was completed by both the patient and a clinician at two time points, all during clinical treatment.
Results
A total of 524 patients were included, 71 of whom were diagnosed with AUD only, 284 with AUD and mild NCD (ARCI) and 169 with AUD, major NCD and fulfilling criteria for Korsakoff’s syndrome (KS).
Conclusions
Cognitive performance improved for all three groups during treatment, sustained abstinence and recovery from AUD. A low memory performance on the MoCA without improvement over time was predictive for KS, while improvement on this domain did not differentiate between AUD and ARCI. Changes in overall cognitive performance and orientation in patients with KS were positively related to changes in everyday cognitive functioning.
Short Summary: Cognitive performance improves during treatment, sustained abstinence and recovery from AUD. Low memory performance without improvement over time predicted KS, while improvement in memory did not differentiate between AUD and ARCI. Changes in overall cognitive performance and orientation in KS patients were positively related to changes in everyday cognitive functioning.
INTRODUCTION
About 30–80% of the people seeking treatment for alcohol use disorder (AUD) have cognitive impairments (Copersino et al., 2009; Bruijnen et al., 2019a). In patients with Korsakoff’s syndrome (KS), cognitive impairments are severe and a hallmark of the disorder. KS in chronic alcoholics is caused by thiamine deficiency, which is an indirect effect of the chronic alcohol use (Arts et al., 2017). Its symptoms include severe memory deficits, confabulations, apathy, disorders of affect, social-cognitive problems and impaired insight into the illness (Arts et al., 2017; Rensen et al., 2017). However, most patients with alcohol-related cognitive impairments (ARCI; Heirene et al., 2018) do not fulfil the criteria for KS, as they have less severe cognitive deficits, which are often overlooked and underdiagnosed by clinicians. ARCI may be the result of indirect effects of alcohol use, such as liver cirrhosis or cerebrovascular risk factors, but may also be caused by direct effects of long-term alcohol abuse in individuals who are not (or not long) abstinent from alcohol, like the toxic actions of alcohol itself or the consequences of alcohol withdrawal. Acute alcohol intoxication primarily acts upon executive functions such as planning, verbal fluency, memory and complex motor control (Peterson et al., 1990; Lyvers and Tobias-Webb, 2010). However, both residual and chronic symptoms of alcohol intoxication are diffuse and found in all cognitive domains (Stavro et al., 2013).
Patients with ARCI themselves do not always report subjective complaints because these may be obscured by the addiction itself, or because of a lack of insight into their own cognitive deficits (Walvoort et al., 2016). In general, the absence of subjective experiences of cognitive deficits is a poor predictor of cognitive performance on objective measures (Horner et al., 1999). In order to detect cognitive impairments in individuals with AUD, cognitive screens can be used that quantify cognitive performance. A relatively short and easy to administer screener is the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005). The MoCA is often used for the detection of ARCI at an early stage of addiction treatment (Bruijnen et al., 2019b) and is being implemented in addiction care more and more (Copersino et al., 2009; Alarcon et al., 2015; Ewert et al., 2018; Ridley et al., 2018). Oudman et al. (2014) found the MoCA to be superior to the Mini-Mental State Examination (Folstein et al., 1975) in distinguishing patients with KS from controls. The availability of three alternate forms of the MoCA makes it possible to retest individuals over time (Chertkow et al., 2011) and thus follow the course of cognitive functioning during treatment. All three versions are found to be largely equivalent and the MoCA total score is a reliable measure for screening cognitive performance (Bruijnen et al., 2020).
The first aim of the present study was to explore the course of cognitive performance on the MoCA during treatment towards abstinence and recovery in three patient groups with AUD: patients with AUD without cognitive impairments, patients with ARCI (but no KS) and patients with KS. It was hypothesized that patients with AUD-only showed the highest overall cognitive performance, followed by patients with ARCI and those with KS, respectively. Furthermore, we expected that between clinical admission, when abstinence is not always guaranteed, and after 6 weeks of admission, all three groups would show an improved cognitive performance, where patients with AUD-only were hypothesized to have a near-ceiling score on the MoCA. Between 6 weeks of admission and clinical discharge, patients with ARCI were expected to have improved further, while cognitive performance in patients with AUD-only and KS was expected to have stayed relatively stable.
The second aim was to explore the course of everyday cognitive functioning in patients with AUD-only, ARCI or KS, as measured with the Patient Competency Rating Scale (PCRS; Prigatano et al., 1986). The PCRS is a rating scale that can be completed by both the patient and a clinician who is familiar with the patient and his/her abilities. The PCRS primarily aims to evaluate an individual’s awareness of cognitive, self-care and social deficits. The possibility to have the questionnaire assessed by both the patient and a clinician makes it possible to map everyday cognitive functioning during treatment from a clinical viewpoint. Everyday cognitive functioning was hypothesized to be best for patients with AUD-only, followed by patients with ARCI and those with KS, respectively. Furthermore, it was hypothesized that everyday cognitive functioning was better at clinical discharge than at 6 weeks of admission in patients with AUD-only and those with ARCI, according to both the patient and the clinician. The reported improvement was expected to be greater according to the clinician than according to the patient.
The third aim was to determine if changes in cognitive performance (MoCA) were related to changes in everyday cognitive functioning (PCRS), between the sixth week of admission and clinical discharge. It was hypothesized that these changes were positively correlated and that the correlations were highest for the clinician ratings.
MATERIALS AND METHODS
Design
A multiple time-series design was used, in which the MoCA was administered at three time points of assessment during clinical treatment. The first administration took place at intake or clinical admission (T0). The second administration followed after approximately 6 weeks of admission (T1). The third and final administration was right before clinical discharge (T2). Data were collected between May 2010 and May 2019 and supplemented from an existing clinical research database. The study was approved by the internal review board of Vincent van Gogh Institute for Psychiatry and all patients provided informed consent in accordance with the Dutch General Data Protection Regulation and the declaration of Helsinki.
Participants
All participants were inpatients of the Centre of Excellence for Korsakoff and Alcohol-Related Cognitive Impairments of Vincent van Gogh Institute for Psychiatry in Venray, The Netherlands. They were referred to the clinic with suspected cognitive impairments related to long-term alcohol use. The patients were diagnosed by a multidisciplinary team in the first 10 to 12 weeks of admission, based on an extensive neuropsychological assessment that was administered after a minimum of 6 weeks abstinence (Walvoort et al., 2013), a neurological and psychiatric examination, observations from therapists and (psychiatric) nurses, physical exams and neuroradiological examination (MRI). Note that the MoCA was not used in this diagnostic process.
Three patient groups were included in the study, all of whom fulfilled the DSM-5 criteria for AUD, with two groups also fulfilling the DSM-5 criteria for mild and major substance-induced (alcohol) neurocognitive disorder (NCD; American Psychiatric Association, 2013). The first group was diagnosed with AUD (not fulfilling the criteria for NCD), the second group with ARCI (fulfilling the criteria for mild NCD) and the third group with KS (fulfilling the criteria for major NCD). The latter group also fulfilled the clinical criteria of KS described by Kopelman (2002) and Arts et al. (2017). These include (a) the presence of a persistent memory impairment resulting in severe deficits in social functioning; (b) the absence of delirium or dementia due to a neurodegenerative disease; (c) evidence for a history of Wernicke encephalopathy; (d) confabulatory behaviour and (e) a history of malnutrition or thiamine deficiency. None of the patients had any evidence of brain abnormalities that could account for their condition apart from atrophy or white-matter lesions associated with chronic alcohol use (Arts et al., 2017), and none of the patients fulfilled the proposed criteria for alcohol-related dementia (Oslin et al., 1998). Finally, none of the included patients had hearing problems, language or communication deficits, or visual deficits that made MoCA administration impossible.
The Centre of Excellence for Korsakoff and Alcohol-Related Cognitive Impairments consist of two separate treatment wards for patients with either ARCI or KS. As patients with AUD-only have no cognitive impairments (after having completed neuropsychological assessment), they are discharged or referred to outpatient aftercare soon after completing the diagnostic process. For the other two groups (ARCI and KS), treatment focuses on reaching the highest level of autonomy in activities of daily living. When treatment is completed, they are also discharged, in most cases to their homes with outpatient aftercare (mostly patients with ARCI), or placed in a long-term residence or sheltered living facility (mostly patients with KS). As these long-term aftercare options have a limited capacity and often have waiting lists, this contributes to a longer stay duration in the patient group who cannot return home.
Measures
Montreal Cognitive Assessment
The MoCA (Nasreddine et al., 2005) consists of 12 items. Scores on all items add up to the MoCA Total Score (MoCA-TS) with a maximum of 30 points, where a higher score represents better cognitive performance. An adjustment for level of education is applied in which participants with a low level of education are awarded two extra points and participants with an average level of education are awarded one extra point, maintaining the maximum score of 30 (Bruijnen et al., 2019b).
Seven Domain Scores (MoCA-DS) were calculated: executive functioning (alternating trail making and verbal fluency; 0–2 points), visuospatial abilities (figure copy and clock drawing; 0–4 points), attention, concentration and working memory (digit span, sustained attention and serial subtraction; 0–6 points), language (animal naming and sentence repetition; 0–5 points), abstract reasoning (0–2 points), memory (0–5 points) and orientation (0–6 points).
Finally, the Memory Index Score (MoCA-MIS) was calculated separately, in which freely recalled words receive three points, words recalled after a category cue receive two points (cued recall) and correct identification after a multiple-choice cue (recognition) receives one point, with a maximum of 15 points (Julayanont et al., 2014).
All three authorized and validated parallel versions of the Dutch MoCA were used in this study (Costa et al., 2012; Nasreddine and Patel, 2016; Bruijnen et al., 2020). Administration of the MoCA takes ~15 min and scoring can mostly be done during administration.
Patient Competency Rating Scale
The PCRS was developed to evaluate an individual’s awareness of cognitive, self-care and social deficits after (traumatic) brain injury (Prigatano et al., 1986). The scale must be administered to the patient and an informant (clinician and/or relative) who is familiar with the patient and his/her abilities. The PCRS contains 30 items in which the respondent is asked to judge how easy or difficult it is (for the patient) to perform a variety of tasks. Each item is rated on a 5-point Likert scale, ranging from 1 (‘cannot do’) to 5 (‘can do with ease’). A total score (PCRS-TS) ranging from 30 to 150 can be obtained, where higher scores represent a higher level of everyday cognitive functioning (Kolakowsky-Hayner et al., 2012). Four domain scores were calculated, measuring activities of daily living (PCRS-ADL; scoring range 8–40), cognitive abilities (PCRS-CO; scoring range 8–40), interpersonal abilities (PCRS-IP; scoring range 7–35) and emotional lability (PCRS-EM; scoring range 7–35; Leathem et al., 1998). The PCRS has a good internal consistency (Cronbach α = 0.87–0.89) and test–retest reliability for all scores range from 0.63 to 0.84, as measured in patients with acquired brain injury (Hellebrekers et al., 2017).
Procedure
Each patient that was referred to the clinic was discussed in a multidisciplinary team to determine if there was a positive indication for clinical admission. As part of the intake procedure, MoCA 7.1 was administered to each patient at intake or preferably in the first week of admission (T0). In the sixth week of admission, one of the psychologists made an appointment with the patient for administration of MoCA 7.2 (T1). Note that the time between intake and clinical admission varied between patients. Therefore, the time between T0 and T1 also varies, namely between 16 and 395 days (M = 62.7, SD = 45.6; see Fig. 1, left panel). When the patient was (soon to be) clinically discharged, another appointment was made for the administration of MoCA 7.3 (T2). The time between T1 and T2 varied based on the duration of clinical admission, namely between 5 and 645 days (M = 124.2, SD = 93.0; see Fig. 1, right panel). The PCRS was completed by the patient at both T1 and T2 and by the primary responsible caregiver of the patient preferably in the same week. Both the MoCA and the PCRS were part of care as usual and were included in the Routine Outcome Monitoring (ROM), among several other questionnaires that are not part of the current study. Relevant demographic information was derived from the electronic patient files.
Fig. 1.
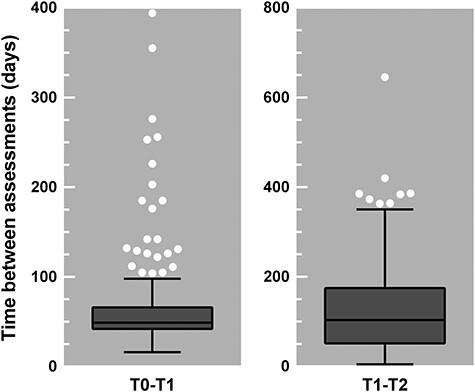
Tukey box-and-whisker plots showing the distribution of the time between two assessments in days. The time between T0 (intake or clinical admission) and T1 (after 6 weeks of clinical admission) ranged from 16 to 394 (left panel), and the time between T1 (after 6 weeks of clinical admission) and T2 (clinical discharge) ranged from 5 to 645 (right panel). The box plots indicate the interquartile range and median; the whiskers indicate the minimum and maximum values, excluding the outliers which are represented by the white circles.
Analyses
The first author thoroughly checked all scores of the MoCA and corrected scoring errors of the assessor when needed. Ambiguities in the scoring for which the instructions on www.mocatest.org were not fully specified were scored or corrected in a consistent manner according to strict criteria (similar to the instructions for the newly released MoCA version 8.1 which was not yet available in Dutch at the time of data collection). The procedure of checking scores of all items by the same assessor eliminated inter-rater differences that were previously found to influence results (Cumming et al., 2020).
First, characteristics of the patient sample as a whole are presented, as well as for all three groups. Differences in patient characteristics between groups were explored using univariate ANOVAs for scaled variables and chi-squared tests for categorical variables.
Second, to explore cognitive performance over the course of treatment, a mixed model ANOVA was used with group (AUD, ARCI and KS) as the between-subject factor and time (T0, T1 and T2) as the within-subject factor. The analysis was run for MoCA-TS, each MoCA-DS and MoCA-MIS, to explore in detail if there are certain domains on which performance changes more than others over the course of treatment.
Third, to explore everyday cognitive functioning over the course of treatment, a mixed-model ANOVA was used with group (AUD, ARCI and KS) as the between-subject factor and time (T1 and T2) as the within-subject factor, ran separately for the patient and clinician ratings. The analyses were run for PCRS-TS and each PCRS-DS, to explore in detail if there are certain domains on which everyday cognitive functioning changes more than others over the course of treatment.
Finally, to explore if changes in cognitive performance were related to changes in everyday cognitive functioning, change scores were calculated between T1 and T2 for all scores (MoCA-TS, all MoCA-DS, MoCA-MIS, PCRS-TS and all PCRS-DS; patient and clinician ratings). Pearson correlations were calculated between the MoCA change scores and the PCRS change scores.
Alpha was set at 0.05 for all main analyses, but to adjust for the Type 1 error rate, Bonferroni corrected, Hochberg’s GT2 (unequal sample sizes) or Games-Howell (non-homogeneous population variances) post hoc tests were used when appropriate. Also, the effect sizes (η2) were calculated and reported based on Lakens (2013). All analyses were performed using IBM SPSS version 25.0.
RESULTS
Patient characteristics
Between June 2010 and March 2019, 796 cases were admitted to the clinic (600 unique patients, as some were readmitted over the years). Of these unique patients, 73.8% were men. The age at admission ranged from 27 to 86 years, with a mean of 56.6 years (SD = 8.7). Of all 796 cases, 91 (11.4%) were diagnosed with AUD, 415 (52.1%) with ARCI and 210 (26.4%) with KS. In the remaining 80 cases (‘other’; 10.1%), 57 were undiagnosed for various reasons, mostly due to leaving the clinic early against medical advice, and another 23 patients had a diagnosis other than AUD, ARCI or KS (i.e. a neurodegenerative disorder [n = 10], non-alcohol or polysubstance use disorder [n = 5], NCD not due to a substance [n = 4], psychotic disorder [n = 2] and depression [n = 2]). Comparisons between these four groups revealed no significant differences for sex distribution, level of education (classified as described by Bruijnen et al., 2019b) and abstinence duration at MoCA administration. We found that patients with KS were significantly older than both patients with AUD and those with ARCI. Duration of admission was shortest for the ‘other’ patients, followed by patients with AUD, ARCI and KS, respectively. Patients with ARCI were significantly more often readmitted to this clinic than all other patient groups (Table 1).
Table 1.
Patient characteristics, means and standard deviations (SDs) for the total sample and split per group. Post hoc comparison gives a description of the direction of significant differences between groups
| Total | AUD | ARCI | KS | Other | P-value | Post hoc comparison | |
|---|---|---|---|---|---|---|---|
| (n = 796) | (n = 91) | (n = 415) | (n = 210) | (n = 80) | |||
| Mean age in years (SD) | 56.63 (8.71) | 54.26 (10.00) | 55.98 (8.22) | 58.65 (7.81) | 57.44 (10.70) | <0.001* | KS > AUD, ARCI |
| Range | 27–86 | 27–76 | 29–78 | 37–77 | 28–86 | ||
| Sex (%)a | 0.375 | ||||||
| Male | 443 (73.8) | 53 (71.6) | 194 (71.1) | 139 (78.1) | 57 (76.0) | ||
| Female | 157 (26.2) | 21 (28.4) | 79 (28.9) | 39 (21.9) | 18 (24.0) | ||
| Level of education (%)b | 0.226† | ||||||
| Unknown | 54 (6.8) | 3 (3.3) | 12 (2.9) | 2 (1.0) | 37 (46.3) | ||
| Low | 203 (25.5) | 26 (28.6) | 107 (25.8) | 58 (27.6) | 12 (15.0) | ||
| Average | 417 (52.4) | 42 (46.2) | 238 (57.3) | 110 (52.4) | 27 (33.8) | ||
| High | 122 (15.3) | 20 (22.0) | 58 (14.0) | 40 (19.0) | 4 (5.0) | ||
| Mean duration of admission in days (SD) | 147.07 (116.57) | 98.08 (61.33) | 132.84 (101.39) | 232.61 (122.53) | 49.67 (82.14) | <0.001* | Other <AUD< ARCI <KS |
| Range | 1–690 | 1–254 | 1–611 | 5–690 | 0–624 | ||
| Mean number of admissions (SD) | 1.44 (1.00) | 1.27 (0.68) | 1.68 (1.24) | 1.17 (0.44) | 1.15 (0.64) | <0.001* | ARCI >AUD, KS, Other |
| Range | 1–9 | 1–5 | 1–9 | 1–4 | 1–5 | ||
| Mean abstinence duration in days (SD) | |||||||
| T0 (n = 299) | 77.37 (570.747) | 17.08 (28.78) | 87.11 (748.34) | 113.82 (460.19) | 8.91 (16.33) | 0.733 | |
| Range | 0–8897 | 0–165 | 0–8897 | 0–3653 | 0–81 | ||
| T1 (n = 463) | 107.68 (468.14) | 59.08 (40.12) | 106.30 (592.74) | 139.75 (367.77) | 57.61 (30.53) | 0.655 | |
| Range | 0–9082 | 5–228 | 0–9082 | 7–3703 | 10–140 | ||
| T2 (n = 313) | 220.04 (541.48) | 123.06 (48.71) | 220.01 (727.17) | 285.24 (268.98) | 79.43 (84.81) | 0.317 | |
| Range | 0–9228 | 51–324 | 0–9228 | 22–2408 | 7–241 | ||
Note: AUD = Alcohol Use Disorder; ARCI = Alcohol-Related Cognitive Impairments; KS=Korsakoff’s Syndrome; Other = undiagnosed or a diagnosis other than AUD, ARCI or KS; T0 = baseline/intake; T1 = after 6 weeks of clinical admission; T2 = at clinical discharge.
aonly unique patients.
bunknown level of education was excluded for group comparisons.
* P < 0.001.
†Fisher’s exact test was used.
Furthermore, in 232 of all 796 cases, the MoCA was not administered, most probably accounted for by: (a) no diagnosis or a diagnosis other than AUD, ARCI or KS; (b) MoCA administration being limited to version 7.1 in the first 2 years of data collection and not being immediately implemented in treatment as usual by all professionals (assessment of MoCA versions 7.2 and 7.3 was introduced in March 2012 and July 2013, respectively); (c) patients being readmitted to the clinic did not complete the MoCA again if they already completed all three versions in their previous admission; (d) patients not being motivated to complete the MoCA assessment and (e) inability to administer the MoCA due to physical limitations or insufficient Dutch language skills.
In the following analyses, only patients with a diagnosis of AUD, ARCI or KS, and at least one MoCA administration were included, to comprise our total sample of 524 patients. Of these, 71 were diagnosed with AUD, 284 with ARCI and 169 with KS. The other 272 patients were excluded. To rule out possible selection bias between the included and excluded cases in terms of demographic characteristics and severity of cognitive impairments, they were statistically compared to the included patients. The distribution of patients between diagnostic groups was unequal, where proportionately more patients with ARCI were excluded, while proportionately more patients with KS were included (χ2(2, n = 716) = 11.55, P = 0.003). There were no differences in age, sex, level of education and abstinence duration at MoCA administration (all P-values >0.05). As expected based on the abovementioned reasons for exclusion, the excluded cases had a significant shorter admission duration than the included cases (Mdiff = −78.15, t(775) = −9.33, P < 0.001) and significantly more readmissions to the clinic (Mdiff = 0.67, t(323.60) = 7.50, P < 0.001).
Course of cognitive performance
There was a significant main effect of time on overall cognitive performance (MoCA-TS; F(1.86,303.83) = 23.39, P < 0.001, η2 = 0.124) and contrasts revealed that this was a significant linear improvement (F(1,163) = 40.25, P < 0.001). There was also a significant main effect of group on cognitive performance (F(2,163) = 34.91, P < 0.001, η2 = 0.300) and post hoc tests showed that differences between all three groups were significant. Patients with AUD scored higher than those with ARCI (Mdiff = 2.55, SD = 0.64) and those with KS (Mdiff = 5.72, SD = 0.70), and patients with ARCI scored higher than those with KS (Mdiff = 3.18, SD = 0.57; all P-values < 0.001). The interaction effect between time and group was not significant (F(3.73,303.83) = 1.03, P = 0.390, η2 = 0.011; see Fig. 2, left top panel).
Fig. 2.
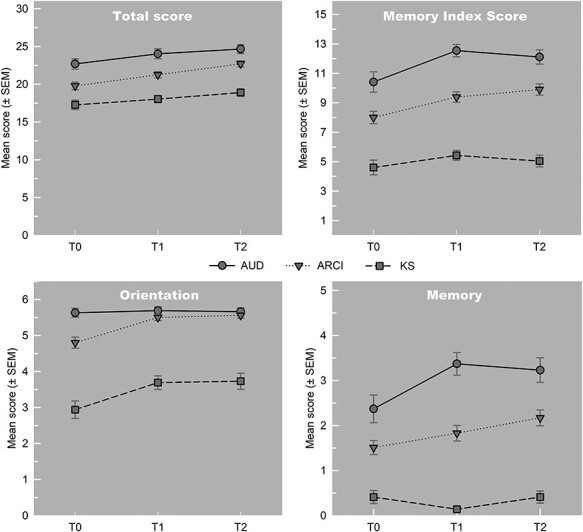
Mean Montreal Cognitive Assessment—Total Score (MoCA-TS; top left panel), Memory Index Score (MoCA-MIS; top right panel), Domain Score orientation (MoCA-DS; bottom left panel) and Domain Score memory (MoCA-DS; bottom right panel) on three assessment time points, split per group.
Significant main effects of time were found for all seven domains (MoCA-DS): executive functioning (F(2,326) = 29.88, η2 = 0.154), visuospatial abilities (F(2,326) = 12.65, η2 = 0.071), attention (F(1.88,306.21) = 5.53, η2 = 0.033), language (F(1.86,303.34) = 46.71, η2 = 0.221), abstract reasoning (F(1.89,308.70) = 23.00, η2 = 0.123), memory (F(1.89,307.78) = 7.94, η2 = 0.044) and orientation (F(1.70,277.71) = 14.17, η2 = 0.078; all P-values ≤ 0.005). The main effect of group on cognitive performance was significant for the domains executive functioning (F(2,163) = 3.83, P = 0.024, η2 = 0.045), memory (F(2,163) = 68.62, P < 0.001, η2 = 0.457) and orientation (F(2,163) = 95.43, P < 0.001, η2 = 0.539). As the directionality of the effects differed between domains, Table 2 provides an outline of significant contrasts and post hoc differences.
Table 2.
Means and SDs of the Montreal Cognitive Assessment Domain Scores (MoCA-DS) on all three assessment points for the total sample and split per group. Post hoc comparison gives a description of the direction of significant differences between groups (column), between assessments (row) and the interaction between groups and assessments (in italics)
| Cognitive domain (range) | Total | AUD | ARCI | KS | P-value | Post hoc comparison |
|---|---|---|---|---|---|---|
| (n = 166) | (n = 35) | (n = 82) | (n = 49) | |||
| Executive functioning (0–2) | 0.024* | AUD > KS† | ||||
| T0 | 0.66 (0.69) | 0.77 (0.73) | 0.65 (0.71) | 0.61 (0.64) | ||
| T1 | 1.02 (0.68) | 1.17 (0.71) | 1.05 (0.70) | 0.88 (0.60) | ||
| T2 | 1.18 (0.74) | 1.43 (0.66) | 1.21 (0.73) | 0.96 (0.76) | ||
| P-value | <0.001*** | 0.499 | ||||
| Post hoc comparison | T0 < T1; T0 < T2; T1 < T2†† | |||||
| Visuospatial abilities (0–4) | 0.066 | |||||
| T0 | 1.97 (1.04) | 2.20 (1.05) | 1.87 (1.00) | 1.98 (1.09) | ||
| T1 | 2.39 (1.07) | 2.63 (0.91) | 2.29 (1.06) | 2.37 (1.17) | ||
| T2 | 2.40 (0.98) | 2.74 (0.74) | 2.41 (1.01) | 2.12 (1.01) | ||
| P-value | <0.001*** | 0.271 | ||||
| Post hoc comparison | T0 < T1;T0 < T2†† | |||||
| Attention (0–6) | 0.063 | |||||
| T0 | 5.00 (1.24) | 5.29 (0.99) | 4.85 (1.30) | 5.04 (1.27) | ||
| T1 | 5.17 (1.12) | 5.31 (1.02) | 5.01 (1.22) | 5.35 (0.99) | ||
| T2 | 5.32 (1.01) | 5.51 (0.82) | 5.13 (1.16) | 5.49 (0.79) | ||
| P-value | 0.005*,††† | 0.819 ††† | ||||
| Post hoc comparison | T0 < T2†† | |||||
| Language (0–5) | 0.538 | |||||
| T0 | 4.07 (0.89) | 4.23 (0.77) | 3.96 (0.87) | 4.14 (1.00) | ||
| T1 | 3.36 (1.01) | 3.51 (1.17) | 3.35 (1.04) | 3.27 (0.84) | ||
| T2 | 3.51 (0.64) | 3.51 (0.66) | 3.50 (0.69) | 3.53 (0.54) | ||
| P-value | <0.001***,††† | 0.440 ††† | ||||
| Post hoc comparison | T0 > T1; T0 > T2†† | |||||
| Abstract reasoning (0–2) | 0.562 | |||||
| T0 | 1.15 (0.78) | 1.20 (0.80) | 1.11 (0.75) | 1.18 (0.81) | ||
| T1 | 1.29 (0.75) | 1.37 (0.69) | 1.20 (0.78) | 1.39 (0.73) | ||
| T2 | 1.66 (0.57) | 1.57 (0.66) | 1.68 (0.54) | 1.69 (0.55) | ||
| P-value | <0.001***,††† | 0.513 ††† | ||||
| Post hoc comparison | T0 < T2; T1 < T2†† | |||||
| Memory (0–5) | <0.001*** | AUD > ARCI; AUD > KS; ARCI>KS†††† | ||||
| T0 | 1.37 (1.57) | 2.37 (1.82) | 1.51 (1.43) | 0.41 (1.02) | ||
| T1 | 1.66 (1.74) | 3.37 (1.48) | 1.83 (1.57) | 0.14 (0.41) | ||
| T2 | 1.87 (1.77) | 3.23 (1.61) | 2.17 (1.60) | 0.41 (0.96) | ||
| P-value | 0.001**,††† | 0.005* ,††† | ||||
| Post hoc comparison | T0 < T1; T0 < T2†† | |||||
| Orientation (0–6) | <0.001*** | AUD > ARCI; AUD > KS; ARCI>KS†††† | ||||
| T0 | 4.43 (1.72) | 5.63 (0.73) | 4.80 (1.40) | 2.94 (1.70) | ||
| T1 | 5.01 (1.27) | 5.69 (0.63) | 5.50 (0.79) | 3.69 (1.31) | ||
| T2 | 5.04 (1.35) | 5.66 (0.64) | 5.56 (0.76) | 3.73 (1.58) | ||
| P-value | <0.001***,††† | 0.052 ††† | ||||
| Post hoc comparison | T0 < T1; T0 < T2†† |
Note: AUD = Alcohol Use Disorder; ARCI = Alcohol-Related Cognitive Impairments; KS=Korsakoff’s Syndrome; T0 = baseline/intake; T1 = after 6 weeks of clinical admission; T2 = at clinical discharge.
†Bonferroni.
††Hochberg’s GT2.
†††Greenhouse–Geisser.
††††Games-Howell.
* P < 0.05;
* * P < 0.005;
* * * P < 0.001.
A significant interaction between time and group on cognitive performance was found for the memory domain (F(3.78,307.78) = 3.86, P = 0.005, η2 = 0.043) and contrasts revealed a significant linear improvement (F(2,163) = 3.08, P = 0.049), as well as a significant quadratic trend (F(2,163) = 5.10, P = 0.007) over time. This means that scores for each group did not change equally over time. As can be seen in Fig. 2, right bottom panel, patients with KS did not change over time and patients with AUD did not change between T1 and T2, while those with ARCI showed linear improvement. Post hoc tests further revealed that differences between all three groups were significant. Patients with AUD scored higher than patients with ARCI (Mdiff = 1.15, SD = 0.24) and those with KS (Mdiff = 2.67, SD = 0.22), and patients with ARCI scored higher than those with KS (Mdiff = 1.52, SD = 0.16; all P-values < 0.001; see Table 2). The interaction between time and group was marginally significant for the orientation domain (F(3.41,277.71) = 2.51, P = 0.052, η2 = 0.028), with contrasts revealing a significant linear improvement over time (F(2,163) = 3.24, P = 0.042), and post hoc tests revealed that differences between all three groups were significant. Patients with AUD scored higher than those with ARCI (Mdiff = 0.37, SD = 0.10) and those with KS (Mdiff = 2.20, SD = 0.18), and patients with ARCI scored higher than those with KS (Mdiff = 1.83, SD = 0.19; all P-values ≤ 0.001; see Table 2 and Fig. 2, left bottom panel).
For MoCA-MIS, significant main effects of both time (F(1.87,289.98) = 12.51, P < 0.001, η2 = 0.073) and group (F(2,155) = 86.67, P < 0.001, η2 = 0.528) were found, with no significant interaction (F(3.74,289.98) = 1.62, P = 0.174, η2 = 0.019). Contrasts revealed a significant linear improvement over time (F(1,155) = 14.77, P < 0.001), as well as a significant quadratic trend (F(1,155) = 8.91, P = 0.003), meaning that not all groups showed the same linear improvement. As can be seen in Fig. 2, right top panel, patients with KS did not change over time, patients with AUD scored lower on T2 than on T1 and patients with ARCI showed linear improvement. Post hoc tests further revealed that differences between all three groups were significant. Patients with AUD scored higher than those with ARCI (Mdiff = 2.61, SD = 0.48) and those with KS (Mdiff = 6.69, SD = 0.47), and patients with ARCI scored higher than those with KS (Mdiff = 4.08, SD = 0.40; all P-values < 0.001).
Course of everyday cognitive functioning
Patient rating: There was a significant main effect of time on overall everyday cognitive functioning (PCRS-TS; F(1,297) = 8.30, P = 0.004, η2 = 0.027), meaning that patients scored higher on T2 than on T1. Neither a significant main effect of group nor a significant interaction was found. For the domain scores, significant main effects of time were found on ADL (F = 1,297) = 9.26, P = 0.003, η2 = 0.030), CO (F(1,297) = 4.02, P = 0.046, η2 = 0.013) and EM (F(1,297) = 7.19, P = 0.008, η2 = 0.023), where all scores improved. A significant main effect of group was found on CO (F(1,297) = 9.30, P < 0.001, η2 = 0.059), where post hoc tests revealed that patients with AUD scored significantly higher than those with ARCI (Mdiff = 2.09, SD = 0.75, P = 0.017) and those with KS (Mdiff = 3.48, SD = 0.81, P < 0.001). There were no significant interaction effects (see Table 3a).
Table 3.
Means and SDs of the Patient Competency Rating Scale domain scores (PCRS-DS) and total score (PCRS-TS) on two assessment points rated by the patient (a) and the clinician (b) for the total sample and split per group. Post hoc comparison gives a description of the direction of significant differences between groups (column), between assessments (row) and the interaction between groups and assessments (in italics)
| (a) Patient rating | ||||||
|---|---|---|---|---|---|---|
| Cognitive domain (range) | Total | AUD | ARCI | KS | P-value | Post hoc comparison† |
| (n = 300) | (n = 53) | (n = 153) | (n = 94) | |||
| Activities of daily living (8–40) | 0.098 | |||||
| T1 | 34.11 (5.50) | 35.38 (5.43) | 33.54 (6.01) | 34.34 (4.50) | ||
| T2 | 35.05 (4.45) | 36.15 (3.82) | 34.90 (4.66) | 34.67 (4.38) | ||
| P-value | 0.003** | 0.174 | ||||
| Cognitive abilities (8–40) | <0.001*** | AUD > ARCI; AUD > KS | ||||
| T1 | 31.73 (5.61) | 34.23 (4.55) | 31.54 (5.67) | 30.62 (5.67) | ||
| T2 | 32.53 (5.13) | 34.34 (4.46) | 32.84 (5.01) | 30.99 (5.31) | ||
| P-value | 0.046* | 0.162 | ||||
| Interpersonal abilities (7–35) | 0.131 | |||||
| T1 | 27.58 (4.88) | 28.45 (4.51) | 27.24 (5.10) | 27.66 (4.68) | ||
| T2 | 27.90 (4.62) | 29.19 (4.22) | 27.63 (4.86) | 27.61 (4.35) | ||
| P-value | 0.128 | 0.435 | ||||
| Emotional lability (7–35) | 0.679 | |||||
| T1 | 24.52 (4.92) | 23.89 (4.67) | 24.43 (4.96) | 25.02 (4.99) | ||
| T2 | 25.19 (4.80) | 24.92 (4.83) | 25.31 (4.99) | 25.13 (4.52) | ||
| P-value | 0.008* | 0.252 | ||||
| Total score (30–150) | 0.130 | |||||
| T1 | 117.94 (18.16) | 121.94 (16.50) | 116.75 (19.09) | 117.64 (17.34) | ||
| T2 | 120.66 (16.51) | 124.60 (15.20) | 120.69 (16.83) | 118.39 (16.42) | ||
| P-value | 0.004** | 0.197 | ||||
| (b) Clinician rating | ||||||
| Cognitive domain (range) | Total | AUD | ARCI | KS | P-value | Post hoc comparison† |
| (n = 348) | (n = 58) | (n = 179) | (n = 111) | |||
| Activities of daily living (8–40) | <0.001*** | AUD > ARCI; AUD > KS; ARCI>KS | ||||
| T1 | 25.83 (7.22) | 30.98 (5.56) | 28.02 (6.24) | 19.59 (4.94) | ||
| T2 | 26.93 (7.37) | 32.34 (5.38) | 29.01 (6.00) | 20.76 (6.07) | ||
| P-value | <0.001*** | 0.870 | ||||
| Cognitive abilities (8–40) | <0.001*** | AUD > ARCI; AUD > KS; ARCI>KS | ||||
| T1 | 24.67 (7.62) | 29.93 (5.75) | 27.53 (6.04) | 17.31 (4.93) | ||
| T2 | 26.02 (7.64) | 32.05 (4.59) | 28.57 (5.78) | 18.77 (6.04) | ||
| P-value | <0.001*** | 0.435 | ||||
| Interpersonal abilities (7–35) | <0.001*** | AUD > KS; ARCI>KS | ||||
| T1 | 23.90 (4.63) | 25.43 (4.33) | 24.71 (4.32) | 21.80 (4.59) | ||
| T2 | 23.13 (5.24) | 25.29 (5.25) | 23.83 (4.91) | 20.88 (4.99) | ||
| P-value | 0.031* | 0.576 | ||||
| Emotional lability (7–35) | 0.162 | |||||
| T1 | 23.44 (4.37) | 23.29 (4.20) | 23.62 (4.28) | 23.23 (4.65) | ||
| T2 | 21.77 (4.77) | 23.16 (4.80) | 21.82 (4.70) | 20.97 (4.74) | ||
| P-value | <0.001*** | 0.032 * | AUD: T1 = T2; ARCI: T1 > T2; KS: T1 > T2; T1: AUD = ARCI=KS; T2: AUD > KS | |||
| Total score (30–150) | <0.001*** | AUD > ARCI; AUD > KS;ARCI>KS | ||||
| T1 | 97.84 (19.67) | 109.61 (16.66) | 103.88 (16.87) | 81.93 (15.09) | ||
| T2 | 97.86 (21.18) | 112.84 (17.17) | 103.22 (17.82) | 81.38 (17.56) | ||
| P-value | 0.493 | 0.266 | ||||
Note: AUD = Alcohol Use Disorder; ARCI = Alcohol-Related Cognitive Impairments; KS=Korsakoff’s Syndrome; T1 = after 6 weeks of clinical admission; T2 = at clinical discharge.
†Hochberg’s GT2.
* P < 0.05.
* * P < 0.005.
* * * P < 0.001.
Clinician rating: There was a significant main effect of group on overall everyday cognitive functioning (PCRS-TS; F(2,345) = 102.30, P < 0.001, η2 = 0.372), where post hoc tests revealed that patients with AUD scored higher than those with ARCI (Mdiff = 7.69, SD = 2.25) and those with KS (Mdiff = 29.59, SD = 2.41), and patients with ARCI scored higher than those with KS (Mdiff = 21.89, SD = 1.80; all P-values < 0.005). Neither a significant main effect of time nor a significant interaction effect was found for overall everyday cognitive functioning. Significant main effects of time were found on all domain scores (ADL: F(1,345) = 15.68, P < 0.001, η2 = 0.043; CO: F(1,345) = 21.37, P < 0.001, η2 = 0.058; IP F(1,345) = 4.68, P = 0.031, η2 = 0.013; EM: F(1,345) = 21.81, P < 0.001, η2 = 0.058) and significant main effects of group were found on ADL (F(2,345) = 120.33, P < 0.001, η2 = 0.411), CO (F(2,345) = 186.69, P < 0.001, η2 = 0.520) and IP (F(2,345) = 25.92, P < 0.001, η2 = 0.131; see Table 3b for directionality of the findings). There was also a significant interaction between time and group on EM (F(2,345) = 3.49, P = 0.032, η2 = 0.019), showing that emotional lability of patients with AUD did not change over time, while both patients with ARCI and those with KS scored lower on T2 than on T1.
Correlation between changes in cognitive performance and everyday cognitive functioning
Changes in overall cognitive performance (MoCA-TS) were positively correlated to changes in overall everyday cognitive functioning (PCRS-TS), as rated by both the patient (r(287) = 0.134, P = 0.012) and the clinician (r(297) = 0.256, P < 0.001).
On an exploratory basis, correlations between all change-scores of the MoCA and the PCRS were calculated for the total sample and for all three groups separately (Table A1a and b in Appendix 1). Main findings were that overall correlations were higher for the clinician rating than for the patient rating and higher for patients with KS followed by those with ARCI and AUD, respectively. For the latter, correlations mostly centred zero. The highest correlations were found in patients with KS, where both the change scores of the MoCA-TS and the MoCA-DS orientation correlated significantly with all PCRS-scores of the clinician.
DISCUSSION
Aims of this study were to explore the course of cognitive performance and subjective everyday cognitive functioning during treatment towards abstinence and recovery in patients with AUD, ARCI and KS in a large clinical sample, and to determine if changes in cognitive performance are related to changes in everyday cognitive functioning. It was found that cognitive performance improved significantly over the course of treatment and differed between groups. Everyday cognitive functioning also improved significantly over time, according to both the patient and the clinician. Significant differences between groups were only found on the clinician rating. For both cognitive performance and everyday cognitive functioning, patients with AUD scored higher than those with ARCI and KS, and patients with ARCI scored higher than those with KS. Finally, changes in overall cognitive performance were positively correlated to changes in overall everyday cognitive functioning.
Overall cognitive performance improved significantly between intake and the sixth week of clinical admission, supporting our hypothesis. In these first 6 weeks, detoxification and recovery are the main goals of treatment. Although neither being abstinent nor abstinence duration was previously found to be related to cognitive performance (Bruijnen et al., 2019b), our findings are in line with the recommendation to perform extended neuropsychological assessment after a minimum of 6 weeks of abstinence, as this seems to be a sufficient period of time for cognitive functioning to recover to a baseline (Walvoort et al., 2013). Particularly in patients with KS, it is argued that cognitive impairments are mostly irreversible and thus may not recover above a ceiling level after abstinence is reached (Arts et al., 2017). When comparing cognitive performance at discharge in our study to findings by Oudman et al. (2014), we find very similar results. In their study that included 30 patients with KS who were in the chronic phase of the syndrome and had been abstinent for a minimum of 6 months, a mean MoCA-TS of 18.1 (SD = 3.9) was found, which is very comparable to our finding of 18.7 (SD = 3.8). We found the improvement of cognitive performance in all three groups between the sixth week of admission and clinical discharge, not supporting our hypothesis that patients with AUD or KS would not improve further during treatment. This means that all patients with AUD can benefit from prolonged clinical treatment. As the time between T1 and T2 varied between patients, additional analyses were performed to examine a possible relation between admission time and cognitive performance, which was not found. Exploration of the domain scores showed that patients with KS did not change on the memory domain, while patients with ARCI improved over all three assessments. Taking the length of clinical stay and the number of readmissions into account, patients with ARCI recover most from short-term clinical treatment. However, these alcohol-related cognitive impairments may increase the risk of readmission (resulting from a relapse into alcohol use), making this the most vulnerable group of patients.
Another finding is that patients rate their own everyday cognitive functioning to be better than that rated by clinicians. While clinician ratings are significantly lower in patients with KS, followed by patients with ARCI and those with AUD, respectively, and thus supporting our hypothesis, patient ratings did not differ between groups. This finding is in line with the literature in which patients do not always report subjective complaints because of a lack of insight into their own cognitive deficits (Walvoort et al., 2016). As opposed to the patient ratings, clinicians do not report a significant change in overall everyday cognitive functioning over the course of treatment, which does not support our hypothesis. This finding can be explained when looking at the four domains separately. As scores for activities of daily living and cognitive abilities improved significantly, scores for interpersonal abilities and emotional lability significantly declined over time. Patients with AUD and ARCI reported significant improvements in overall everyday cognitive functioning, thus partly supporting our hypothesis, and also on the domains activities of daily living, cognitive abilities and emotional lability. These changes may be influenced by the fact that patients were probably in a better emotional state when completing the second assessment, as they were (soon to be) clinically discharged.
Although several significant positive correlations were found between changes in cognitive performance and changes in everyday cognitive functioning, the effect sizes remained mostly small to medium. This supports the literature that cognitive performance on objective measures is not predictive for cognitive deficits in the absence of subjective experiences of these deficits (Horner et al., 1999). Correlations were highest between overall cognitive performance and the clinician ratings of everyday cognitive functioning. Interestingly, changes in cognitive performance on orientation also correlated significantly with everyday cognitive functioning and correlations were overall higher for patients with KS. These findings on correlations between cognitive performance and everyday cognitive functioning partly support our hypothesis.
There are several strengths to this study. First, being able to include a large group of patients and follow them over a significant amount of time in an inpatient setting, makes the findings highly clinically relevant and generalizable to the population. Second, due to an extensive (multidisciplinary) diagnostic process, it was possible to compare three well-described patient groups. Third, because the patients were clinically admitted information from multiple sources, including from clinicians who were familiar with the patient and his/her abilities, could be included. Finally, two instruments (MoCA and PCRS) were used that are freely available and are easy to administer.
A limitation to the study is that not all patients who were admitted to the clinic during data collection could be included. Despite the fact that almost one-third of patients were excluded from the study, we strongly argue that this group does not represent a subsample of patients. As is explained in detail in the Participants section, exclusion was mostly based on the lack of implementation of the MoCA in the first few years of the study, readmission of patients during the study or early discharge against medical advice. The results also showed that the included patients were still representative for the total sample.
In summary, this study describes the course of cognitive performance on the MoCA during treatment towards abstinence and recovery, in three patient groups. The study confirms that patients with AUD had the highest MoCA scores, followed by patients with ARCI and those with KS, respectively. Surprisingly, all three groups improved significantly over time. It can be concluded that performance on the memory domain is the best predictor for KS: scores were significantly lowest and no improvement occurred in the first 6 weeks of abstinence and recovery, where patients with AUD and those with ARCI scored higher and improved over the course of treatment. As for everyday cognitive functioning, it was confirmed that patients have a lack of insight into their cognitive deficits, as scores of all three patient groups were comparable while the clinician reports were significantly different between groups. Interestingly, by comparing changes in cognitive performance to changes in everyday cognitive functioning, it was found that especially for patients with KS, changes in overall cognitive performance and on the domain orientation relate positively to changes in everyday cognitive functioning.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGEMENTS
The authors like to thank all colleagues at the Centre of Excellence for Korsakoff and Alcohol-Related Cognitive Impairments of the Vincent van Gogh Institute for Psychiatry, The Netherlands, for their contributions to administering, scoring and interpreting the MoCA, and their hard work in rating the PCRS for each patient. A special thank you goes out to Jeanine Mijnders-Noeverman, Laura van Boxtel and Iris Aaftink. We would also like to thank Julia Birke and Anniek van de Lustgraaf for their contributions in data entry.
Appendix 1: Correlations between change scores of the MoCA and the PCRS
Table A1.
Correlations between the change scores of the Montreal Cognitive Assessment Domain Scores (MoCA-DS), Total Score (MoCA-TS) and Memory Index Score (MoCA-MIS), and the change scores of the Patient Competency Rating Scale domain scores (PCRS-DS) and Total Score (PCRS-TS) between the sixth week of admission (T1) and at clinical discharge (T2) for the patient (a) and clinician (b) ratings for the total sample and split per group.
| (a) Patient rating | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 287) | AUD (n = 51) | ARCI (n = 149) | KS (n = 87) | |||||||||||||||||
| MoCA | PCRS | PCRS | PCRS | PCRS | ||||||||||||||||
| ADL | CO | IP | EM | TS | ADL | CO | IP | EM | TS | ADL | CO | IP | EM | TS | ADL | CO | IP | EM | TS | |
| EF | −0.02 (0.395) | −0.01 (0.448) | −0.03 (0.294) | 0.01 (0.418) | −0.01 (0.413) | −0.04 (0.399) | −0.15 (0.155) | −0.15 (0.152) | −0.31 (0.013)* | −0.22 (0.065) | −0.02 (0.408) | −0.06 (0.234) | −0.06 (0.232) | −0.00 (0.848) | −0.04 (0.296) | 0.00 (0.496) | 0.14 (0.107) | 0.07 (0.251) | 0.18 (0.047)* | 0.12 (0.130) |
| VA | 0.15 (0.006)* | 0.05 (0.190) | 0.03 (0.314) | 0.01 (0.436) | 0.08 (0.100) | 0.13 (0.190) | −0.06 (0.330) | −0.16 (0.131) | −0.17 (0.110) | −0.08 (0.282) | 0.19 (0.011)* | 0.11 (0.087) | 0.05 (0.294) | 0.00 (0.496) | 0.11 (0.098) | 0.09 (0.211) | −0.01 (0.482) | 0.05 (0.315) | 0.07 (0.251) | 0.06 (0.283) |
| ACW | 0.05 (0.179) | 0.12 (0.019)* | 0.07 (0.124) | 0.09 (0.069) | 0.11 (0.038)* | 0.06 (0.328) | 0.02 (0.450) | −0.02 (0.445) | −0.03 (0.426) | 0.02 (0.455) | −0.02 (0.425) | 0.11 (0.101) | 0.07 (0.209) | 0.10 (0.104) | 0.08 (0.167) | 0.17 (0.062) | 0.20 (0.034)* | 0.11 (0.154) | 0.11 (0.159) | 0.19 (0.042)* |
| L | 0.08 (0.088) | 0.08 (0.101) | 0.12 (0.018)* | 0.03 (0.320) | 0.09 (0.056) | 0.19 (0.088) | −0.11 (0.230) | 0.02 (0.454) | −0.22 (0.061) | −0.04 (0.401) | 0.04 (0.304) | 0.09 (0.131) | 0.10 (0.107) | 0.04 (0.307) | 0.09 (0.151) | 0.11 (0.158) | 0.13 (0.119) | 0.24 (0.012)* | 0.12 (0.132) | 0.18 (0.047)* |
| AR | −0.03 (0.326) | 0.09 (0.062) | −0.02 (0.390) | −0.01 (0.443) | 0.02 (0.388) | −0.13 (0.182) | −0.12 (0.210) | −0.14 (0.170) | −0.13 (0.187) | −0.18 (0.109) | 0.06 (0.239) | 0.19 (0.011)* | 0.05 (0.272) | 0.10 (0.124) | 0.12 (0.068) | −0.17 (0.062) | −0.05 (0.317) | −0.10 (0.171) | −0.16 (0.067) | −0.15 (0.085) |
| M | 0.06 (0.172) | 0.00 (0.499) | −0.02 (0.382) | −0.03 (0.334) | 0.01 (0.464) | 0.09 (0.259) | −0.13 (0.184) | −0.19 (0.090) | −0.31 (0.013)* | −0.17 (0.111) | 0.09 (0.138) | 0.03 (0.376) | 0.03 (0.339) | 0.04 (0.332) | 0.06 (0.251) | −0.08 (0.229) | −0.02 (0.414) | −0.05 (0.325) | −0.00 (0.490) | −0.05 (0.327) |
| O | 0.12 (0.024)* | 0.12 (0.021)* | 0.09 (0.073) | 0.07 (0.124) | 0.12 (0.019)* | 0.07 (0.323) | −0.24 (0.045)* | −0.31 (0.013)* | −0.11 (0.217) | −0.20 (0.079) | 0.11 (0.092) | 0.14 (0.047)* | 0.06 (0.240) | 0.11 (0.095) | 0.13 (0.062) | 0.17 (0.063) | 0.21 (0.026)* | 0.25 (0.009)* | 0.09 (0.204) | 0.22 (0.019)* |
| TS | 0.15 (0.006)* | 0.14 (0.009)* | 0.08 (0.089) | 0.05 (0.184) | 0.13 (0.012)* | 0.12 (0.198) | −0.19 (0.087) | −0.25 (0.039)* | −0.36 (0.005)* | −0.22 (0.060) | 0.17 (0.019)* | 0.20 (0.007)* | 0.11 (0.087) | 0.13 (0.061) | 0.19 (0.011)* | 0.14 (0.101) | 0.22 (0.022)* | 0.23 (0.018)* | 0.15 (0.080) | 0.23 (0.017)* |
| MIS | 0.04 (0.272) | 0.06 (0.172) | 0.01 (0.437) | 0.02 (0.400) | 0.04 (0.260) | 0.10 (0.241) | −0.16 (0.129) | −0.23 (0.052) | −0.35 (0.006)* | −0.21 (0.073) | 0.02 (0.400) | 0.02 (0.403) | 0.03 (0.341) | 0.03 (0.379) | 0.03 (0.359) | 0.03 (0.405) | 0.19 (0.043)* | 0.05 (0.318) | 0.12 (0.135) | 0.12 (0.125) |
| (b) Clinician rating | ||||||||||||||||||||
| Total (n = 297) | AUD (n = 52) | ARCI (n = 154) | KS (n = 91) | |||||||||||||||||
| MoCA | PCRS | PCRS | PCRS | PCRS | ||||||||||||||||
| ADL | CO | IP | EM | TS | ADL | CO | IP | EM | TS | ADL | CO | IP | EM | TS | ADL | CO | IP | EM | TS | |
| EF | 0.06 (0.151) | 0.12 (0.023)* | 0.05 (0.186) | 0.09 (0.060) | 0.10 (0.040)* | −0.04 (0.398) | −0.03 (0.423) | 0.01 (0.479) | 0.14 (0.164) | 0.03 (0.418) | 0.08 (0.174) | 0.16 (0.026)* | 0.07 (0.206) | 0.04 (0.311) | 0.11 (0.091) | 0.09 (0.204) | 0.12 (0.123) | 0.06 (0.304) | 0.14 (0.092) | 0.14 (0.101) |
| VA | 0.12 (0.022)* | 0.20 (<0.001)*** | 0.07 (0.116) | 0.13 (0.011)* | 0.17 (0.002)** | 0.10 (0.244) | 0.22 (0.057) | 0.03 (0.415) | 0.27 (0.029)* | 0.20 (0.074) | 0.15 (0.033)* | 0.19 (0.009)* | 0.13 (0.049)* | 0.20 (0.006)* | 0.21 (0.005)** | 0.07 (0.272) | 0.21 (0.022)* | −0.04 (0.370) | −0.07 (0.272) | 0.06 (0.283) |
| ACW | 0.12 (0.020)* | 0.12 (0.017)* | 0.07 (0.105) | 0.01 (0.407) | 0.10 (0.036)* | 0.18 (0.099) | −0.06 (0.346) | 0.10 (0.233) | 0.13 (0.182) | 0.11 (0.210) | 0.11 (0.096) | 0.18 (0.014)* | 0.05 (0.254) | −0.04 (0.293) | 0.10 (0.118) | 0.12 (0.129) | 0.10 (0.178) | 0.09 (0.200) | 0.05 (0.321) | 0.12 (0.136) |
| L | 0.08 (0.082) | 0.13 (0.015)* | 0.03 (0.277) | −0.01 (0.445) | 0.08 (0.097) | 0.13 (0.183) | 0.08 (0.300) | −0.22 (0.056) | −0.13 (0.173) | −0.05 (0.354) | 0.11 (0.086) | 0.21 (0.004)** | 0.20 (0.007)* | 0.03 (0.361) | 0.17 (0.016)* | 0.03 (0.408) | 0.02 (0.419) | −0.08 (0.226) | 0.03 (0.388) | −0.00 (0.500) |
| AR | 0.02 (0.359) | 0.14 (0.008)* | 0.16 (0.003)** | 0.08 (0.085) | 0.13 (0.013)* | −0.11 (0.223) | −0.03 (0.417) | 0.01 (0.470) | 0.02 (0.441) | −0.03 (0.410) | 0.02 (0.392) | 0.10 (0.099) | 0.14 (0.040)* | 0.04 (0.302) | 0.10 (0.116) | 0.09 (0.198) | 0.31 (0.001)** | 0.30 (0.002)** | 0.22 (0.018)* | 0.31 (0.002)** |
| M | 0.08 (0.084) | 0.04 (0.265) | −0.02 (0.385) | −0.02 (0.352) | 0.02 (0.338) | 0.32 (0.011)* | 0.11 (0.225) | 0.00 (0.497) | 0.16 (0.129) | 0.19 (0.091) | 0.04 (0.299) | 0.03 (0.356) | −0.04 (0.307) | −0.05 (0.251) | −0.01 (0.477) | 0.02 (0.421) | 0.03 (0.391) | 0.07 (0.266) | −0.04 (0.338) | 0.02 (0.44) |
| O | 0.21 (<0.001)*** | 0.21 (<0.001)*** | 0.16 (0.003)** | 0.15 (0.005)* | 0.23 (<0.001)*** | 0.17 (0.110) | 0.10 (0.244) | −0.00 (0.498) | 0.20 (0.279) | 0.15 (0.139) | 0.16 (0.028)* | 0.18 (0.013)* | 0.11 (0.081) | −0.01 (0.451) | 0.14 (0.044)* | 0.30 (0.002)** | 0.30 (0.002)** | 0.28 (0.004)** | 0.32 (0.001)** | 0.39 (<0.001)*** |
| TS | 0.23 (<0.001)*** | 0.30 (<0.001)*** | 0.16 (0.004)** | 0.13 (0.015)* | 0.26 (<0.001)*** | 0.25 (0.039)* | 0.12 (0.192) | −0.02 (0.456) | 0.21 (0.069) | 0.18 (0.097) | 0.22 (0.003)** | 0.33 (<0.001)*** | 0.20 (0.008)* | 0.06 (0.242) | 0.25 (<0.001)*** | 0.25 (0.008)* | 0.36 (<0.001)*** | 0.21 (0.022)* | 0.21 (0.022)* | 0.34 (<0.001)*** |
| MIS | 0.08 (0.085) | 0.02 (0.377) | −0.01 (0.465) | −0.03 (0.323) | 0.02 (0.365) | 0.21 (0.064) | 0.07 (0.309) | −0.01 (0.474) | 0.18 (0.101) | 0.15 (0.148) | −0.00 (0.496) | −0.05 (0.263) | −0.08 (0.171) | −0.06 (0.224) | −0.06 (0.235) | 0.18 (0.047)* | 0.14 (0.097) | 0.13 (0.108) | −0.05 (0.330) | 0.13 (0.113) |
Note: AUD = Alcohol Use Disorder; ARCI = Alcohol-Related Cognitive Impairments; KS=Korsakoff’s Syndrome; ADL = Activities of Daily Living; CO=Cognitive abilities; IP=Interpersonal abilities; EM = Emotional lability; TS = Total Score; EF = Executive Functioning; VA = Visuospatial Abilities; ACW = Attention, Concentration and Working memory; L = Language; AR = Abstract Reasoning; M = Memory; O=Orientation.
* P < 0.05;
* * P < 0.005;
* * * P < 0.001.
Contributor Information
C J W H Bruijnen, Center of Excellence for Korsakoff and Alcohol-Related Cognitive Disorders, Vincent van Gogh Institute for Psychiatry, 5800 Venray, The Netherlands; Nijmegen Institute for Scientist-Practitioners in Addiction (NISPA), Radboud University, 6500 Nijmegen, The Netherlands; Donders Institute for Brain, Cognition and Behaviour, Radboud University, 6500 Nijmegen, The Netherlands.
S J W Walvoort, Center of Excellence for Korsakoff and Alcohol-Related Cognitive Disorders, Vincent van Gogh Institute for Psychiatry, 5800 Venray, The Netherlands; Nijmegen Institute for Scientist-Practitioners in Addiction (NISPA), Radboud University, 6500 Nijmegen, The Netherlands.
B A G Dijkstra, Nijmegen Institute for Scientist-Practitioners in Addiction (NISPA), Radboud University, 6500 Nijmegen, The Netherlands; Novadic-Kentron, Addiction Care Center, 5260 Vught, The Netherlands.
C A J de Jong, Nijmegen Institute for Scientist-Practitioners in Addiction (NISPA), Radboud University, 6500 Nijmegen, The Netherlands; Behavioural Science Institute, Radboud University, 6500 Nijmegen, The Netherlands.
R P C Kessels, Center of Excellence for Korsakoff and Alcohol-Related Cognitive Disorders, Vincent van Gogh Institute for Psychiatry, 5800 Venray, The Netherlands; Donders Institute for Brain, Cognition and Behaviour, Radboud University, 6500 Nijmegen, The Netherlands; Department of Medical Psychology, Radboud University Medical Center, 6500 Nijmegen, The Netherlands.
FUNDING
This study was funded by the Vincent van Gogh Institute for Psychiatry, Venray, The Netherlands and the Nijmegen Institute for Scientist-Practitioners in Addiction (NISPA), Nijmegen, The Netherlands.
CONFLICT OF INTEREST STATEMENT
No potential conflict of interest was reported by the authors.
References
- Alarcon R, Nalpas B, Pelletier S, et al. (2015) MoCA as a screening tool of neuropsychological deficits in alcohol-dependent patients. Alcohol Clin Exp Res 39:1042–8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Arts NJM, Walvoort SJW, Kessels RPC (2017) Korsakoff's syndrome: a critical review. Neuropsychiatr Dis Treat 13:2875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnen CJWH, Dijkstra BAG, Walvoort SJW, et al. (2019a) Prevalence of cognitive impairment in patients with substance use disorder. Drug Alcohol Rev 38:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnen CJWH, Dijkstra BAG, Walvoort SJW, et al. (2020) Psychometric properties of the Montreal Cognitive Assessment (MoCA) in healthy participants aged 18-70. Int J Psychiat Clin 24:293–300. [DOI] [PubMed] [Google Scholar]
- Bruijnen CJWH, Jansen M, Dijkstra BAG, et al. (2019b) The Montreal Cognitive Assessment (MoCA) as a cognitive screen in addiction health care: a validation study for clinical practice. J Subst Use 24:47–54. [Google Scholar]
- Chertkow H, Nasreddine ZS, Johns E, et al. (2011) The Montreal Cognitive Assessment (MoCA): validation of alternate forms and new recommendations for education corrections. Alzheimers Dement 7:S157. [Google Scholar]
- Copersino ML, Fals-Stewart W, Fitzmaurice G, et al. (2009) Rapid cognitive screening of patients with substance use disorders. Exp Clin Psychopharmacol 17:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AS, Fimm B, Friesen P, et al. (2012) Alternate-form reliability of the Montreal Cognitive Assessment screening test in a clinical setting. Dement Geriatr Cogn Disord 33:379–84. [DOI] [PubMed] [Google Scholar]
- Cumming TB, Lowe D, Linden T, et al. (2020) The AVERT MoCA data: scoring reliability in a large multicenter trial. Assessment 27(5):976–81. [DOI] [PubMed] [Google Scholar]
- Ewert V, Pelletier S, Alarcon R, et al. (2018) Determination of MoCA cutoff score in patients with alcohol use disorders. Alcohol Clin Exp Res 42:403–12. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR (1975) Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–98. [DOI] [PubMed] [Google Scholar]
- Heirene R, John B, Roderique-Davies G (2018) Identification and evaluation of neuropsychological tools used in the assessment of alcohol-related cognitive impairment: a systematic review. Front Psychol 9:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellebrekers D, Winkens I, Kruiper S, et al. (2017) Psychometric properties of the awareness questionnaire, Patient Competency Rating Scale and dysexecutive questionnaire in patients with acquired brain injury. Brain Inj 31:1469–78. [DOI] [PubMed] [Google Scholar]
- Horner MD, Harvey RT, Denier CA (1999) Self-report and objective measures of cognitive deficit in patients entering substance abuse treatment. Psychiatry Res 86:155–61. [DOI] [PubMed] [Google Scholar]
- Julayanont P, Brousseau M, Chertkow H, et al. (2014) Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc 62:679–84. [DOI] [PubMed] [Google Scholar]
- Kolakowsky-Hayner SA, Wright J, Bellon K (2012) A brief overview of the Patient Competency Rating Scale: Updates and additions to the COMBI. J Head Trauma Rehabil 27:83–5. [DOI] [PubMed] [Google Scholar]
- Kopelman MD (2002) Disorders of memory. Brain 125:2152–90. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathem JM, Murphy LJ, Flett RA (1998) Self- and informant-ratings on the Patient Competency Rating Scale in patients with traumatic brain injury. J Clin Exp Neuropsychol 20:694–705. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Tobias-Webb J (2010) Effects of acute alcohol consumption on executive cognitive functioning in naturalistic settings. Addict Behav 35:1021–8. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Patel BB (2016) Validation of Montreal Cognitive Assessment, MoCA, alternate French versions. Can J Neurol Sci 43:665–71. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–9. [DOI] [PubMed] [Google Scholar]
- Oslin D, Atkinson RM, Smith DM, et al. (1998) Alcohol related dementia: proposed clinical criteria. Int J Geriatr Psychiatry 13:203–12. [DOI] [PubMed] [Google Scholar]
- Oudman E, Postma A, Van der Stigchel S, et al. (2014) The Montreal Cognitive Assessment (MoCA) is superior to the mini mental state examination (MMSE) in detection of Korsakoff's syndrome. Clin Neuropsychol 28:1123–32. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, et al. (1990) Acute alcohol intoxication and cognitive functioning. J Stud Alcohol 51:114–22. [DOI] [PubMed] [Google Scholar]
- Prigatano GP, Fordyce D, Zeiner H, et al. (1986) Neuropsychological Rehabilitation after Brain Injury. Baltimore, MD: Johns Hopkins Univrsity Press. [Google Scholar]
- Rensen YC, Oosterman JM, Walvoort SJW, et al. (2017) Intrusions and provoked and spontaneous confabulations on memory tests in Korsakoff's syndrome. J Clin Exp Neuropsychol 39:101–11. [DOI] [PubMed] [Google Scholar]
- Ridley N, Batchelor J, Draper B, et al. (2018) Cognitive screening in substance users: diagnostic accuracies of the mini-mental state examination, Addenbrooke’s cognitive examination–revised, and Montreal Cognitive Assessment. J Clin Exp Neuropsychol 40:107–22. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S (2013) Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol 18:203–13. [DOI] [PubMed] [Google Scholar]
- Walvoort SJW, Van der Heijden PT, Kessels RPC, et al. (2016) Measuring illness insight in patients with alcohol-related cognitive dysfunction using the Q8 questionnaire: a validation study. Neuropsychiatr Dis Treat 12:1609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walvoort SJW, Wester AJ, Egger JIM (2013) Neuropsychologische diagnostiek en cognitieve functies bij alcoholabstinentie. Tijdschr Psychiatr 55:101–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


