Figure 1. Role of Diet and Phospholipase D enzyme in the complex network of choline pathways and Choline-Mediated 1C metabolism.
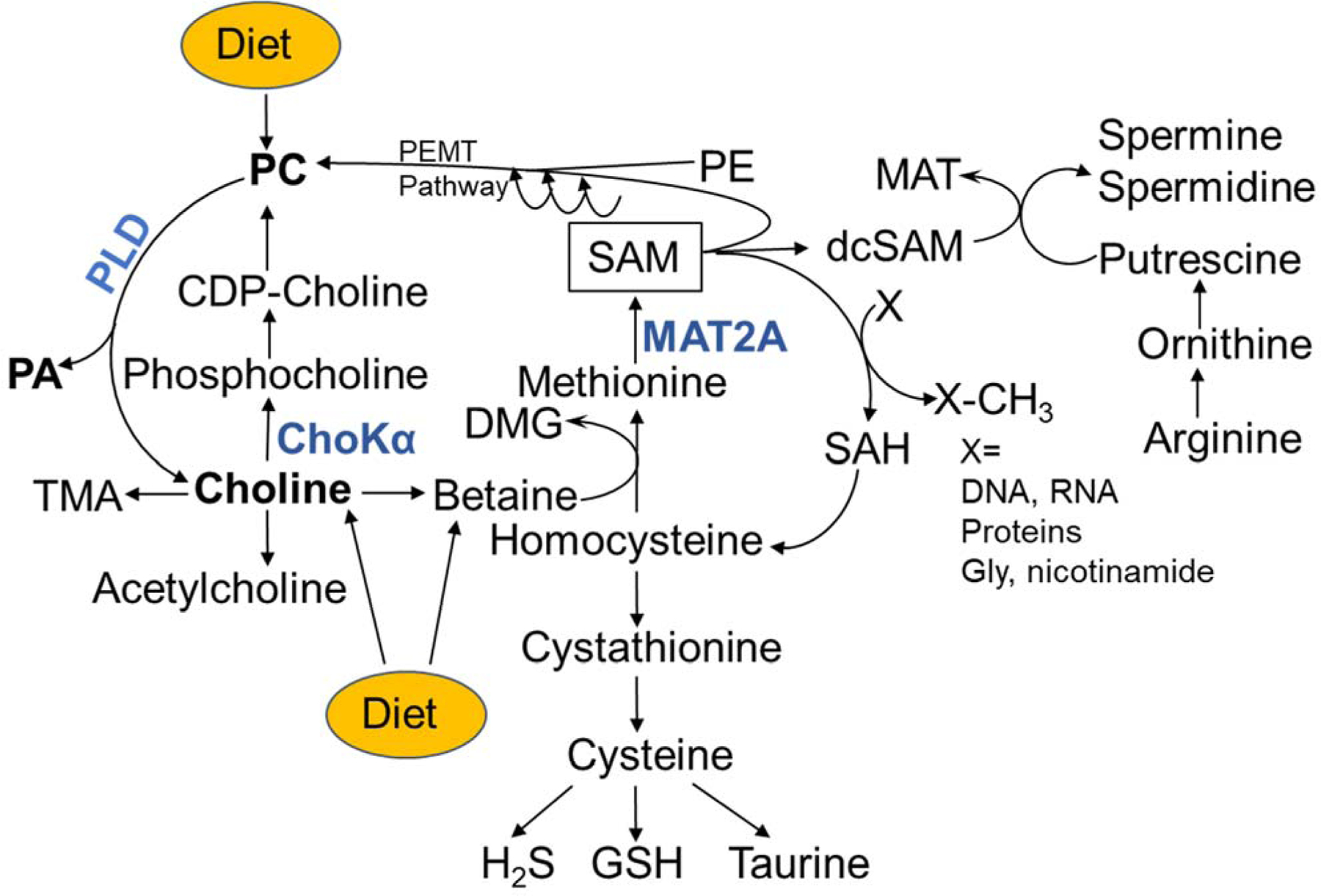
Phospholipase D (PLD) catalyzes release of choline from either diet-derived or endogenously synthesized phosphatidylcholine (PC). The free choline product of PLD activity, or obtained from diet, is a substrate for the synthesis of acetylcholine, trimethylamine (TMA), and phosphatidylcholine (PC) via Cytidine diphosphate choline (CDP-choline). Formation of phosphocholine catalyzed by choline kinase α (ChoKα) is the initial step of de novo PC synthesis. Choline can also be oxidized to betaine. The oxidation of choline to betaine links PLD enzyme activity to 1C metabolism through de novo synthesis of methionine from homocysteine. The byproduct of this reaction dimethylglycine (DMG) is also a potential methyl donor. The methionine product is utilized for synthesis of S-adenocylmethionine (SAM) catalyzed by methionine adenosyltransferase 2A (MAT2A). SAM is the universal methyl donor for a wide range of substrates including phosphatidylethanolamine (PE), DNA, RNA and proteins including histones, and generating S-adenosylhomocysteine (SAH). SAH is hydrolyzed to homocysteine which couples 1C metabolism to the transsulfuration pathway involving sequential synthesis of cystathionine and cysteine and ultimately forming glutathione, taurine and hydrogen sulfide (H2S). SAM can also be decarboxylated to decarboxylated S-adenosylmethionine (dc-SAM) which supports synthesis of the polyamines, spermine and spermidine. The byproduct 50 -methylthioadenosine (MTA) is salvaged back to methionine for SAM generation. The highlighted enzymes PLD, ChoKα and MAT2A are presently attractive therapeutic targets for treatment of cancer.
