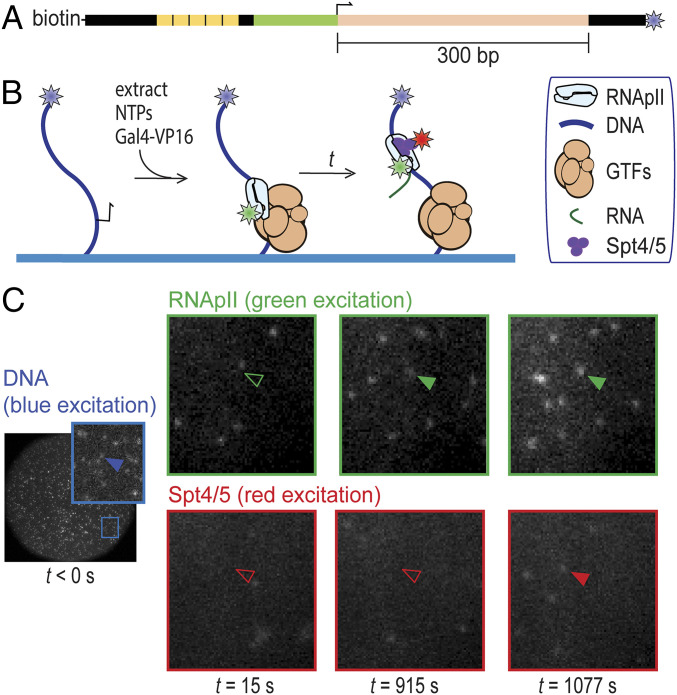
Fig. 1.
Detection of RNApII and Spt4/5 binding to individual surface-tethered DNA488 molecules in Rpb1SNAP549/Spt5DHFR-Cy5 S. cerevisiae nuclear extract. (A) Schematic of DNA488 transcription template. This DNA contains five upstream Gal4 binding sites (yellow) and the CYC1 core promoter (green) with its transcription start site (bent arrow), followed by a 300-bp cassette (pink) encoding a G-less RNA. The template has attached biotin and AF488 dye (blue star) moieties. (B) Experimental scheme. DNA488 molecules immobilized on the surface of a flow chamber (blue) were at time t = 0 incubated with yeast nuclear extract containing dye (stars)-labeled proteins Rpb1SNAP549 and Spt5DHFR-Cy5 along with unlabeled general transcription factors (GTFs) and other nuclear proteins. Reactions were supplemented with recombinant Gal4-VP16 activator. RNApII and Spt4/5 binding to DNA were detected as colocalization of spots of green- and red-excited fluorescence at locations of blue-excited DNA spots. (C) Images of the same microscope field of view (65 × 65 µm) in the red-, green-, and blue-excited fluorescence channels taken at various times before (blue) and after (red and green) extract addition at time t = 0. Insets show magnified views of the marked region. Absence or presence of a spot of fluorescence colocalized with a particular DNA molecule are shown by open and filled arrowheads, respectively.